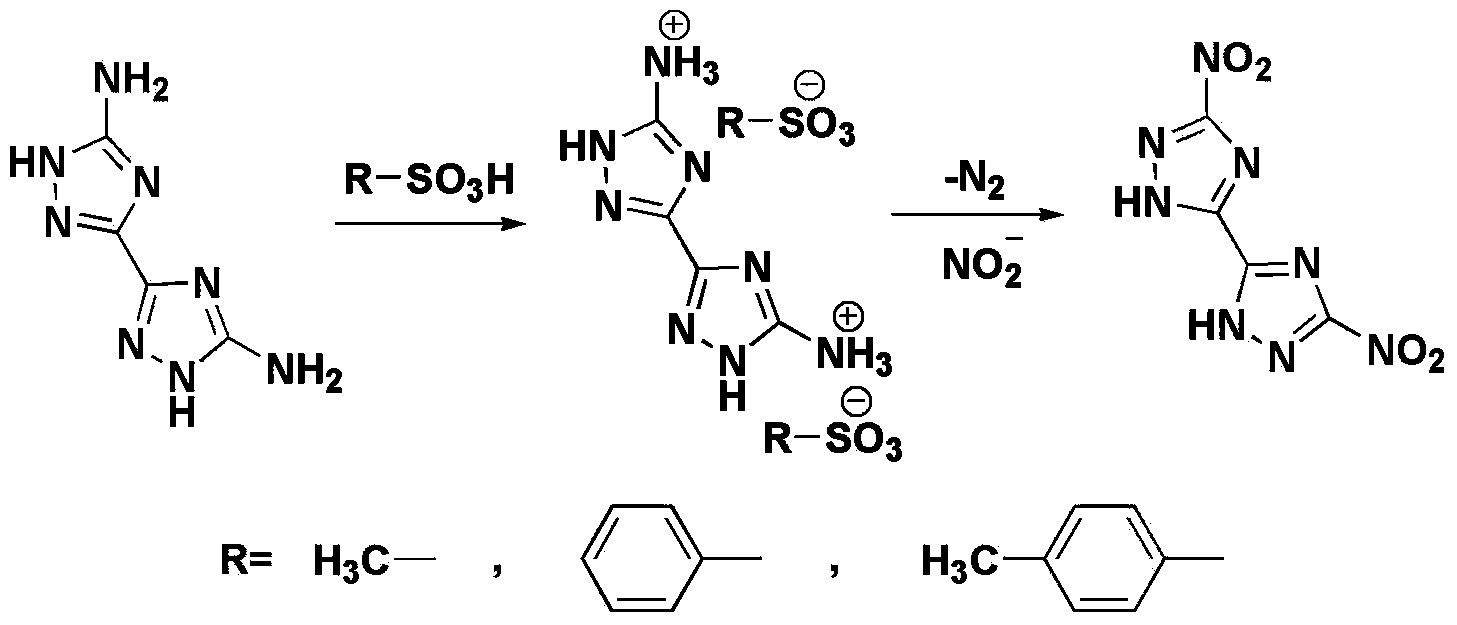
Synthetic method of 3,3'-binitro-5,5'-di-1,2,4-triazole
A synthesis method, dinitro technology, applied in the direction of organic chemistry, can solve the problems of increased side reactions, difficult operation, and high consumption of sodium nitrite, and achieve less reaction by-products, high reaction yield, and simple post-treatment process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
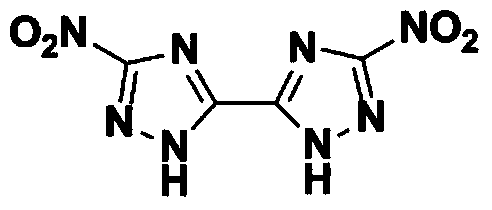
[0023] Dissolve 4.0 g of 5,5'-diamino-3,3'-bi-1,2,4-triazole in 32.0 g of methanesulfonic acid to prepare a salt solution for use. Add 50mL aqueous solution containing 11.6g of sodium nitrite to a reaction flask equipped with stirring. Add the above-mentioned salt solution dropwise to the reaction flask at a temperature of 20°C. Control the dropping time to 30min. After the salt solution is added dropwise, The water bath is heated to 40°C. At this time, a large amount of gas is released in the system. After stirring for 60 minutes, the gas is released completely. Cool to a temperature of 20°C and filter. The filter cake obtained is washed and dried to obtain 3,3'-dinitro-5. ,5'-Bi-1,2,4-triazole dihydrate 5.85g, purity 98.8%, yield 92.1%.
[0024] Structure Identification:
[0025] Proton nuclear magnetic resonance spectrum: (DMSO-d 6 , 500MHz, δ, ppm): 5.14 (s, br, 2H, NH); carbon nuclear magnetic resonance spectrum: (DMSO-d 6 , 125MHz): 146.6(C-C), 163.0(C-NO 2 )
[0026] Infrare...
Embodiment 2
[0033] Dissolve 4.0 g of 5,5'-diamino-3,3'-bi-1,2,4-triazole in 32.0 g of methanesulfonic acid to prepare a salt solution for use. Add 50mL aqueous solution containing 8.3g of sodium nitrite to a reaction flask equipped with stirring. Add the above-mentioned salt solution dropwise to the reaction flask at a temperature of 20°C. Control the dropping time to 30min. After the salt solution is added dropwise, The water bath is heated to 40°C. At this time, a large amount of gas is released in the system. After stirring for 60 minutes, the gas is released completely. Cool to a temperature of 20°C and filter. The filter cake obtained is washed and dried to obtain 3,3'-dinitro-5. ,5'-Bi-1,2,4-triazole dihydrate 5.36g, purity 98.2%, yield 84.4%.
Embodiment 3
[0035] Dissolve 4.0 g of 5,5'-diamino-3,3'-bi-1,2,4-triazole in 32.0 g of methanesulfonic acid to prepare a salt solution for use. Add a 50mL aqueous solution containing 16.6g of sodium nitrite to a reaction flask equipped with stirring. Add the above-mentioned salt solution dropwise to the reaction flask at a temperature of 20°C. Control the dropping time to 30min. After the salt solution is added dropwise, The water bath is heated to 40°C. At this time, a large amount of gas is released in the system. After stirring for 60 minutes, the gas is released completely. Cool to a temperature of 20°C and filter. The filter cake obtained is washed and dried to obtain 3,3'-dinitro-5. ,5'-Bi-1,2,4-triazole dihydrate 5.77g, purity 98.5%, yield 90.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com