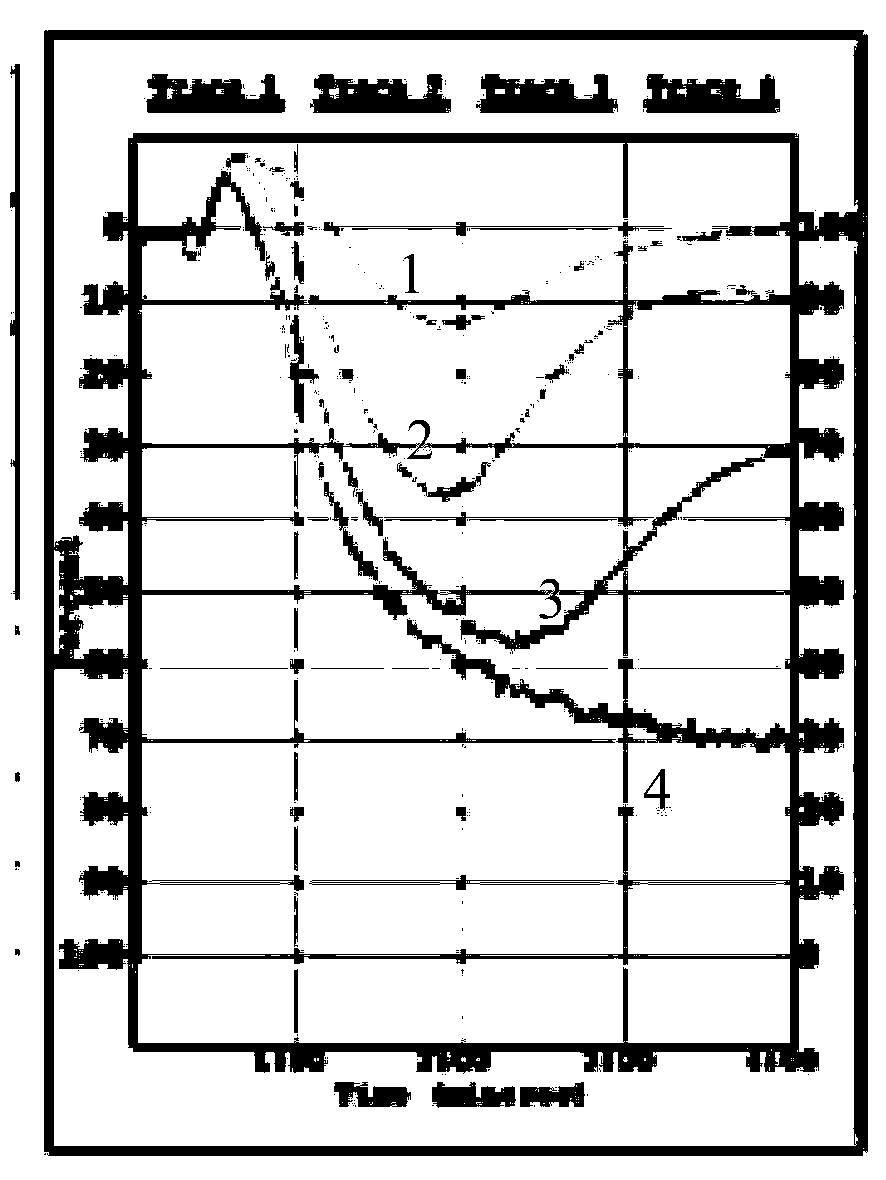
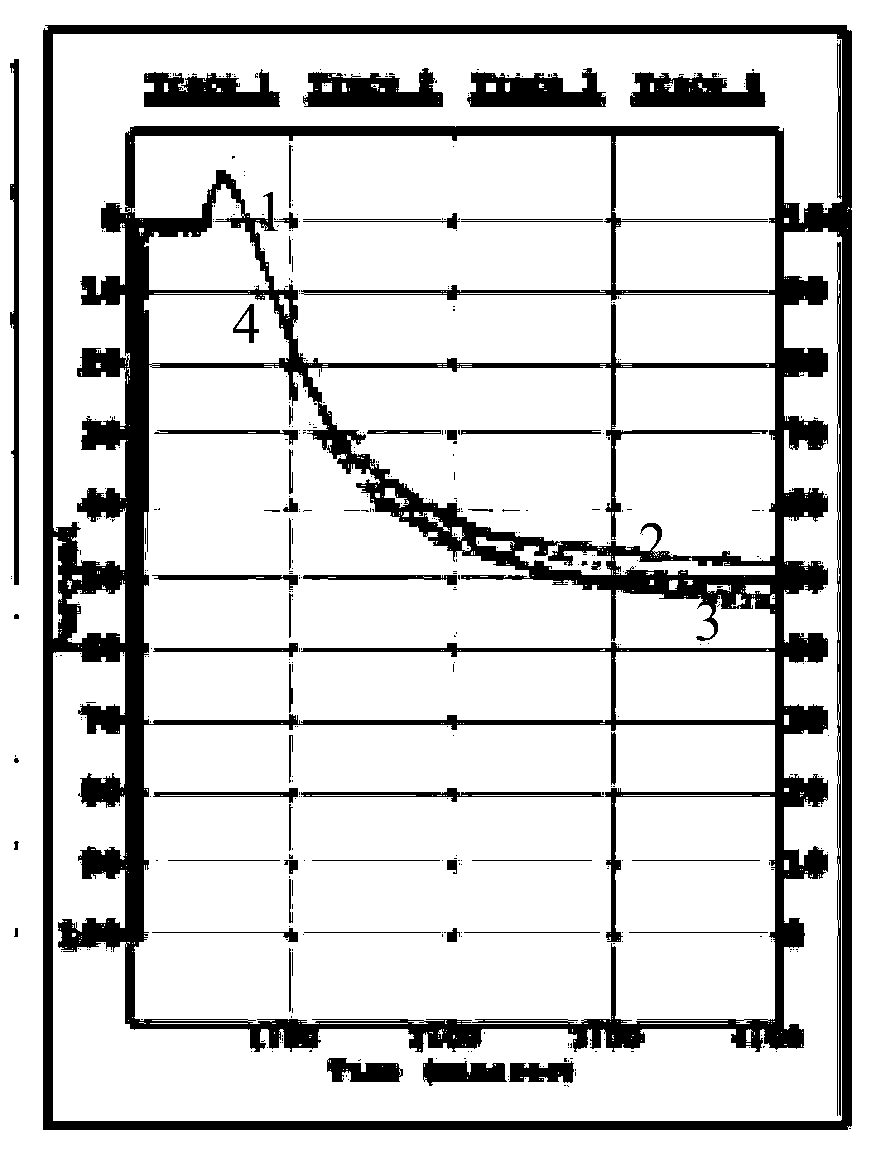
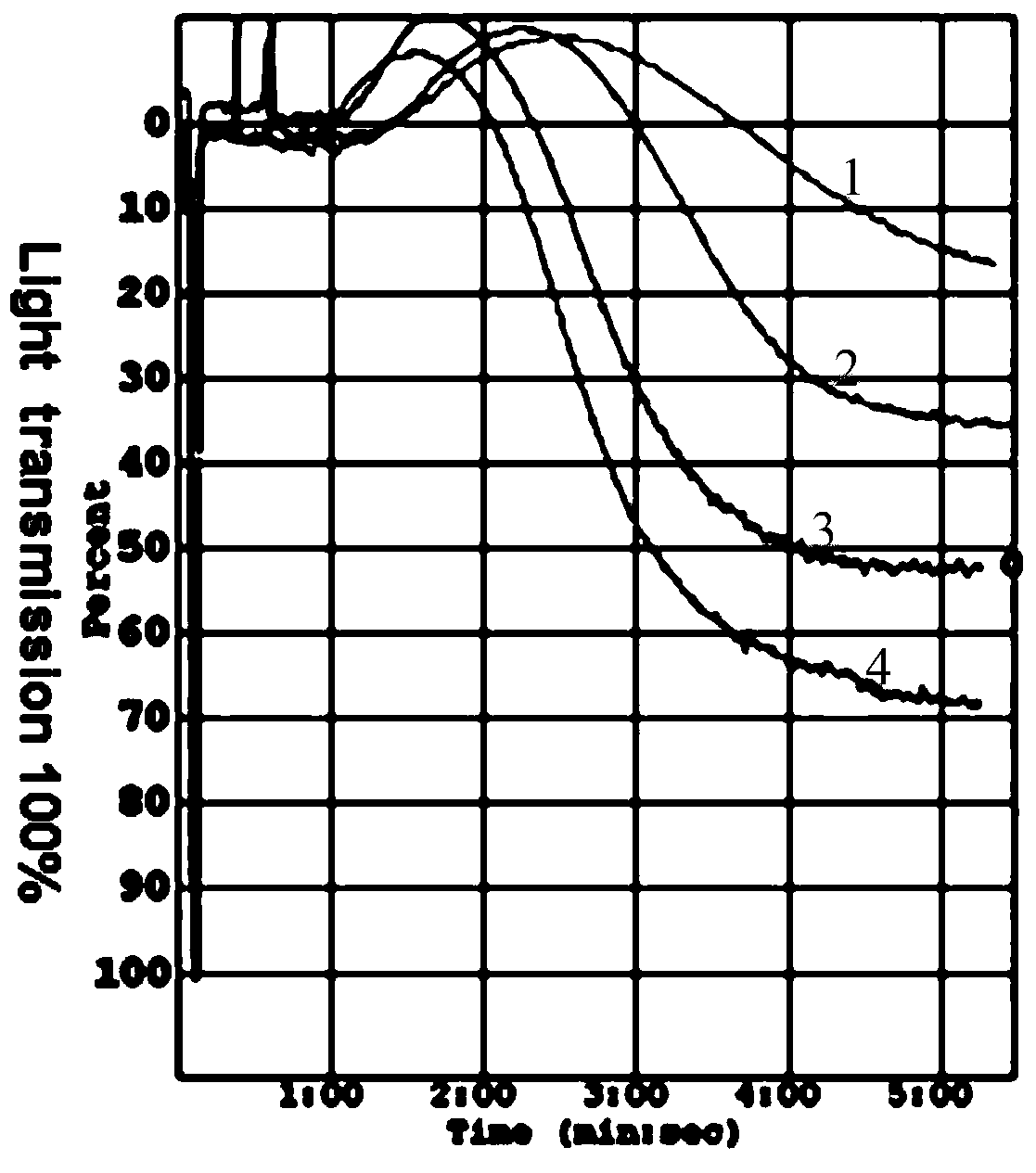
Hybridoma cell, monoclonal antibody generated by hybridoma cell and application of monoclonal antibody
A technology of hybridoma cells and monoclonal antibodies, applied in the field of monoclonal antibodies, to achieve the effect of inhibiting blood coagulation reaction and inhibiting thrombus formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of monoclonal antibody hybridoma cells
[0042] 1. Material
[0043] Antigen: the amino acid sequence protein shown in SEQ ID NO:1, fully synthesized by the reagent company;
[0044] Test animals: BALB / C mice aged 8-10 weeks, about 17g;
[0045] Reagents: Freund's complete adjuvant and incomplete adjuvant (purchased from Promega);
[0046] Myeloma cells: SP2 / 0 cells (provided by the China Type Culture Collection);
[0047] Medium: HAT selective medium (purchased from Sigma);
[0048] 2. Method
[0049] Take 100 μg of the protein of the amino acid sequence shown in SEQ ID NO: 1 plus equal mass Freund’s complete adjuvant for the first subcutaneous injection of BALB / C mice, 21 days later, take 300 μg of the protein of the amino acid sequence of SEQ ID NO: 1 plus The second subcutaneous injection of the same quality Freund’s incomplete adjuvant was carried out to BALB / C mice. After 10 days, blood was collected from the tail and the ELISA indirect method was used ...
Embodiment 2
[0051] Example 2: Secreted production of DuIn antibody
[0052] After immunizing BALB / C mice with incomplete adjuvant for 1 to 2 weeks, they were injected 1×10 into the abdominal cavity of the mice. 6 A hybridoma cell with a deposit number of CGMCC NO. 9153, which produces ascites 7-10 days after inoculation with the hybridoma cell;
[0053] The ascites was extracted from the abdominal cavity of the BALB / C mice, placed in a 15ml centrifuge tube, and centrifuged at 2000 rpm for 10 min to precipitate blood cells and other impurities. The supernatant was transferred to a new centrifuge tube, stored in a -80°C refrigerator, and purified Before, use 0.1M PBS solution to dilute ascites at a ratio of 1:3;
[0054] Take 0.5ml Protein G into the adsorption column, add 20 times the column volume of 0.1M PBS buffer to equilibrate the column for sample loading;
[0055] Take the diluted ascites and add it to the purification column, each time 2-3ml, the ascites circulate through the column 3 time...
Embodiment 3
[0059] Example 3: DuIn antibody specific detection
[0060] Take ERp57fl / fl (control mice, the gene encoding the amino acid sequence protein shown in SEQ ID NO:1 is not knocked out) and PF4-Cre / ERp57fl / fl (platelet specific knock out the amino acid sequence shown in SEQ ID NO:1 Protein-encoding gene mouse) platelet lysate 200μg and ERp57 recombinant protein 50ng were subjected to polyacrylamide gel electrophoresis, then the protein was transferred to PVDF membrane and sealed with 5% skimmed milk solution at room temperature for 1 hour. Use TBS / T solution to dilute the anti-DuIn antibody to 1 μg / ml, put the blocked PVDF membrane into the antibody diluent, and incubate at room temperature for 2 hours. After the incubation, wash with TBS / T solution 3 times for 10 minutes each time, then add membrane to the fluorescently labeled secondary antibody dilution, and incubate at room temperature for 1 hour. After 1 hour, wash the membrane 3 times with TBS / T solution and observe with Odyss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com