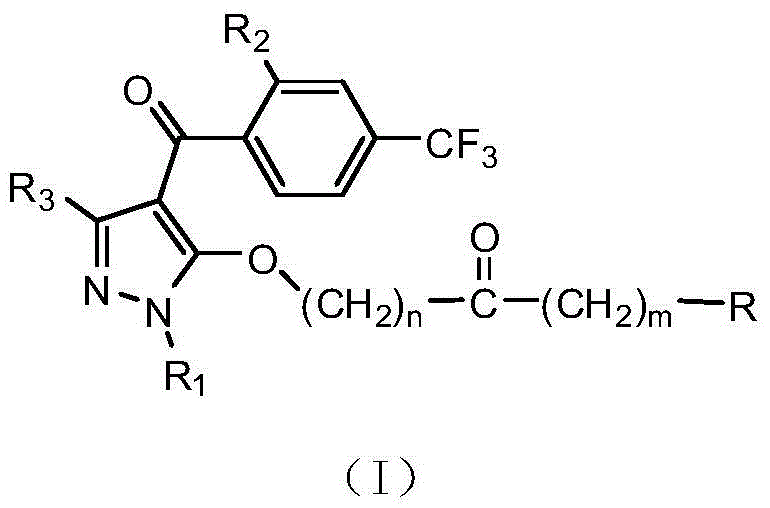
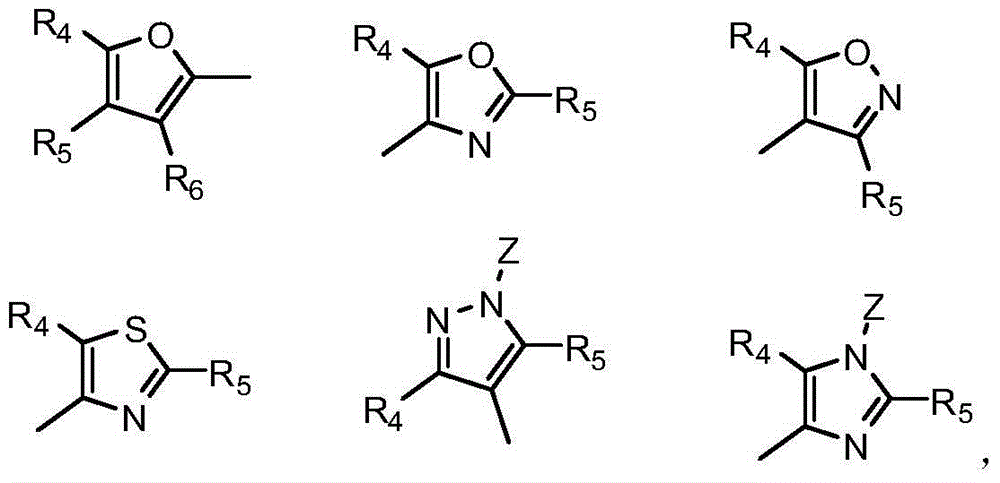
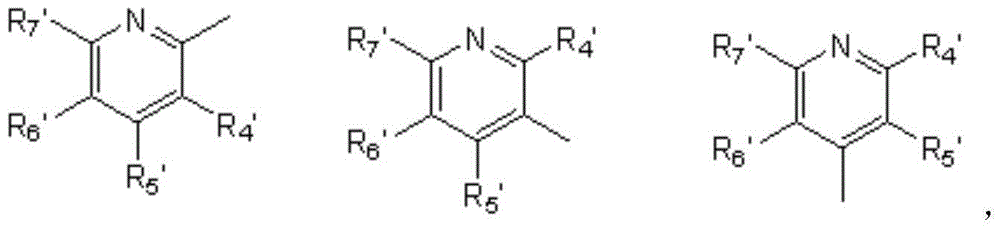
4-benzoyl pyrazole compound with herbicidal activity
A technology of benzoylpyrazoles and herbicidal activity is applied in the directions of organic chemistry, herbicides and algicides, chemicals used for biological control, etc., and can solve the problems of insufficient herbicidal activity and crop safety, etc., achieve good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The synthesis of 4-(2-methylsulfonyl-4-trifluoromethylphenyl)-1,3-dimethyl-5-ethoxyacyloxypyrazole (compound number 001) is as follows:
[0047]
[0048] Add 2.5 g (0.022 mol) of 1,3-dimethyl-5-hydroxypyrazole and 30 mL of toluene into a three-necked flask, and add 9.0 g of triethylamine (0.09 mol) while stirring. Control the temperature in an ice bath at 5-10°C, add dropwise a toluene solution of 6.3g (0.022mol) of 2-methylsulfonyl-4-trifluoromethylbenzoyl chloride, and control the reaction temperature not to exceed 15°C. Ice-water bath, stirred at room temperature for 30min, TLC detection reaction (ethyl acetate:petroleum ether=4:1, GF254, UV color development), after the reaction is complete, add 0.2g of 2-methyl-2-hydroxypropionitrile, stir Slowly heat up to 45-50°C for reaction, TLC detection reaction (ethyl acetate:petroleum ether=2:1, GF254, UV color development), after the reaction is complete, cool to room temperature, dropwise add ethyl chloroformate 2.5g (...
Embodiment 2
[0049]Example 2: 4-(2-methylsulfonyl-4-trifluoromethylphenyl)-1-methyl-3-trifluoromethyl-5-(2-chloronicotinoyloxy)pyrazole (compound number 002 ), the synthetic reaction formula is as follows:
[0050]
[0051] Add 3.0 g (0.018 mol) of 1-methyl-3-trifluoromethyl-5-hydroxypyrazole and 30 mL of acetonitrile into a three-necked flask, and add 8.0 g of triethylamine (0.08 mol) while stirring. Control the temperature in an ice bath at 5-10°C, add dropwise acetonitrile solution of 5.0g (0.018mol) of 2-methylsulfonyl-4-trifluoromethylbenzoyl chloride, control the reaction temperature not to exceed 15°C, remove the Ice-water bath, stirred at room temperature for 30min, TLC detection reaction (ethyl acetate:petroleum ether=4:1, GF254, UV color development), after the reaction is complete, add 0.1g of sodium cyanide, and slowly heat up to 35-45°C under stirring Reaction, TLC detection reaction (ethyl acetate:petroleum ether=2:1, GF254, UV color development), after the reaction is co...
Embodiment 3
[0052] Example 3: 4-(2-methylsulfonyl-4-trifluoromethylphenyl)-1-methyl-3-difluoromethyl-5-(4-methoxyphenoxyacetoxy)pyrazole ( Synthesis of Compound No. 003)
[0053] The synthetic reaction formula is as follows:
[0054]
[0055] Add 3.0 g (0.02 mol) of 1-methyl-3-difluoromethyl-5-hydroxypyrazole and 50 mL of dichloromethane into a three-necked flask, and add 9.0 g of triethylamine (0.09 mol) while stirring. Control the temperature in an ice bath at 5-10°C, add dropwise a solution of 5.7g (0.02mol) of 2-methylsulfonyl-4-trifluoromethylbenzoyl chloride in dichloromethane, control the reaction temperature not to exceed 15°C, and complete the dropwise addition , remove the ice-water bath, stir at room temperature for 30 minutes, TLC detection reaction (ethyl acetate:petroleum ether=4:1, GF254, UV color development), after the reaction is complete, add 0.2g of 2-methyl-2-hydroxypropionitrile, While stirring, the temperature was slowly raised to reflux reaction, and the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com