Polyvinyl alcohol fluorescence fiber film, and making method and application thereof
A technology of polyvinyl alcohol fibers and polyvinyl alcohol, which is applied in the field of fluorescent sensing materials, can solve the problems that conjugated polymer sensing materials cannot be reused, and achieve good reusability, improved stability, and synthetic methods easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
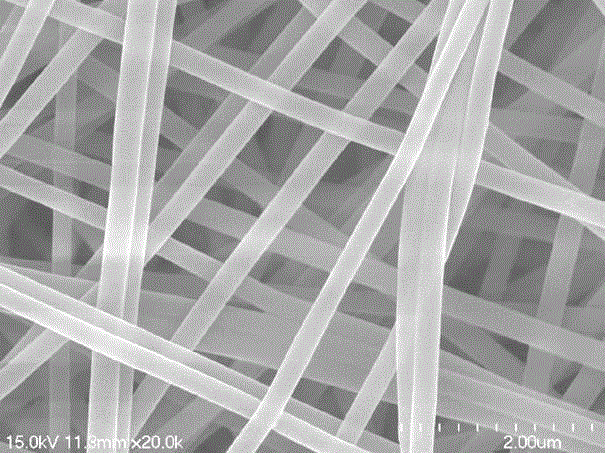
[0027] This embodiment provides a fluorescent fiber membrane that responds to non-polar aromatic hydrocarbons, and its preparation steps and responsiveness testing process are as follows:
[0028] 1. Preparation of precursor aqueous solution
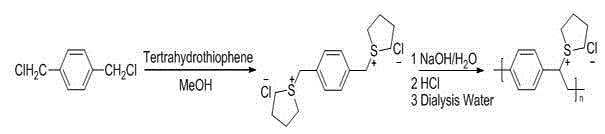
[0029] See attached figure 1 , which is the synthesis route of the precursor in this example, adding 2 parts of tetrahydrothiophene groups to 1 part of p-dichlorobenzyl, and eliminating part of the tetrahydrothiophene groups in the resulting product under the action of sodium hydroxide to obtain the precursor body.
[0030] Refer to literature (J. Am. Chem. Soc1993, 115, 10117-10124; European Polymer Journal 43 (2007) 802-807) to synthesize 1.0038g (about 2.86mmol) of monomer without side group under anaerobic conditions, dissolve it Slowly inject 2mL of sodium hydroxide aqueous solution (about 1.4mol / L) into 16mL of deionized water in an ice-water bath, keep stirring in an ice-water bath for 1 hour after injection, and then put the mi...
Embodiment 2
[0048] This embodiment provides a fluorescent fiber membrane that responds to non-polar aromatic hydrocarbons, and its preparation steps and responsiveness testing process are as follows:
[0049] 1. Preparation of precursor aqueous solution
[0050] See attached figure 1 , which is the synthetic route of the precursor in this example, first add two molecules of tetrahydrothiophene groups to one molecule of p-dichlorobenzyl, and then remove part of the tetrahydrothiophene groups in the resulting product under the action of sodium hydroxide , to obtain the precursor.
[0051] Under anaerobic conditions, 1.0038g (approximately 2.86mmol) of the monomer without side groups synthesized by referring to the literature (J. Am. Chem. Soc1993, 115, 10117-10124; European Polymer Journal 43 (2007) 802-807) was dissolved in Slowly inject 2mL of sodium hydroxide aqueous solution (about 1.4mol / L) into 16mL of deionized water in an ice-water bath. After the injection is completed, keep stir...
Embodiment 3
[0061] This embodiment provides a fluorescent fiber membrane that responds to non-polar aromatic hydrocarbons, and its preparation steps and responsiveness testing process are as follows:
[0062] 1. Preparation of precursor aqueous solution
[0063] See attached figure 1 , which is the synthetic route of the precursor in this example, first add two molecules of tetrahydrothiophene groups to one molecule of p-dichlorobenzyl, and then remove part of the tetrahydrothiophene groups in the resulting product under the action of sodium hydroxide , to obtain the precursor.
[0064] Under anaerobic conditions, 1.0038g (approximately 2.86mmol) of the monomer without side groups synthesized by referring to the literature (J. Am. Chem. Soc1993, 115, 10117-10124; European Polymer Journal 43 (2007) 802-807) was dissolved in Slowly inject 2mL of sodium hydroxide aqueous solution (about 1.4mol / L) into 16mL of deionized water in an ice-water bath. After the injection is completed, keep stir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com