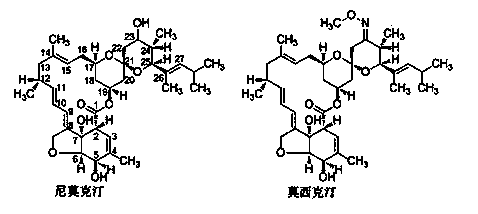
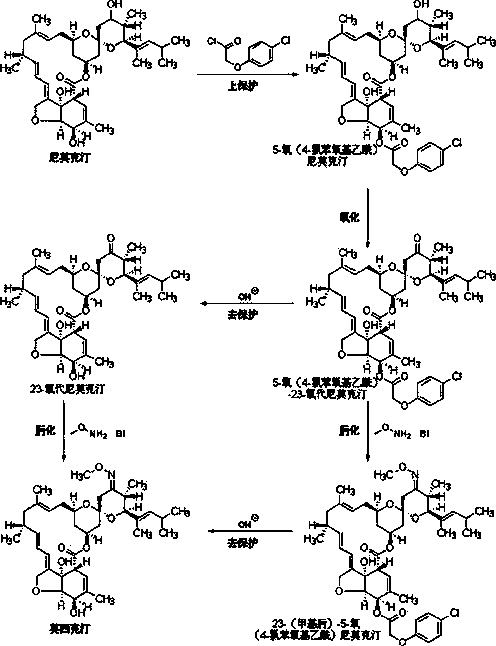
Method of chemically synthesizing moxidectin
A technology for chemical synthesis and nimoctin, applied in organic chemistry and other directions, can solve the problems of potential safety hazards, toxic nitrobenzene compounds, poor thermal stability, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of 5-oxo(4-chlorophenoxyacetyl)nimoctin
[0023] The 1.0L three-necked bottle is equipped with a mechanical stirrer, a thermometer and a constant pressure dropping funnel. Put in nimoctine (58.5g, purity 65%, 41.7mmol, 1.0eq), dichloromethane (333 g) and triethylamine (21.1 g, 208.3mmol, 5.0eq), and stir until dissolved. A solution of 4-chlorophenoxyacetyl chloride (20.5 g, 100 mmol, 2.4 eq) in dichloromethane (222 g) was added dropwise, and the internal temperature was controlled at 20°C-25°C. After the reaction is completed, the reaction solution is washed with 1% hydrochloric acid and 15% saline in sequence, the organic phase is separated and dried by adding anhydrous magnesium sulfate, filtered, and the filtrate is precipitated under negative pressure to obtain 5-oxo(4-chlorophenoxyacetyl)nemo Crude cretin was 60.8g (HPLC purity 56.9%).
[0024] Get the crude product of 5-oxo(4-chlorophenoxyacetyl)nemoctine and recrystallize it with methanol...
Embodiment 2
[0026] Example 2 Preparation of 5-oxo(4-chlorophenoxyacetyl)nimoctin
[0027] The 0.1L three-necked bottle is equipped with mechanical stirring, thermometer and constant pressure dropping funnel. Put in nimoctine (5.9g, purity 65%, 4.1mmol, 1.0eq), dichloromethane (30g) and pyridine (1.6g, 20.5mmol, 5.0eq), and stir until it dissolves. A solution of 4-chlorophenoxyacetyl chloride (5.5 g, 16.6 mmol, 4 eq) in dichloromethane (20 g) was added dropwise, and the internal temperature was controlled at 25°C-30°C. After the reaction is completed, the reaction solution is washed with 1% hydrochloric acid and 15% saline in sequence, the organic phase is separated and dried by adding anhydrous magnesium sulfate, filtered, and the filtrate is precipitated under negative pressure to obtain 5-oxo(4-chlorophenoxyacetyl)nemo Crude cretin 5.8g (HPLC purity 52.4%).
Embodiment 3
[0028] Example 3 Preparation of 5-oxo(4-chlorophenoxyacetyl)nimoctin
[0029] The 0.1L three-necked bottle is equipped with mechanical stirring, thermometer and constant pressure dropping funnel. Put in nimoctine (5.0g, purity 90%, 6.9mmol, 1.0eq), dichloromethane (30g) and triethylamine (2.1g, 20.7mmol, 3.0eq), and stir until dissolved. A solution of 4-chlorophenoxyacetyl chloride (2.8 g, 13.8 mmol, 2 eq) in dichloromethane (10 g) was added dropwise, and the internal temperature was controlled at 20°C-25°C. After the reaction is completed, the reaction solution is washed with 1% hydrochloric acid and 15% saline in sequence, the organic phase is separated and dried by adding anhydrous magnesium sulfate, filtered, and the filtrate is precipitated under negative pressure to obtain 5-oxo(4-chlorophenoxyacetyl)nemo 6.1 g of crude cretin (HPLC purity: 88.9%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com