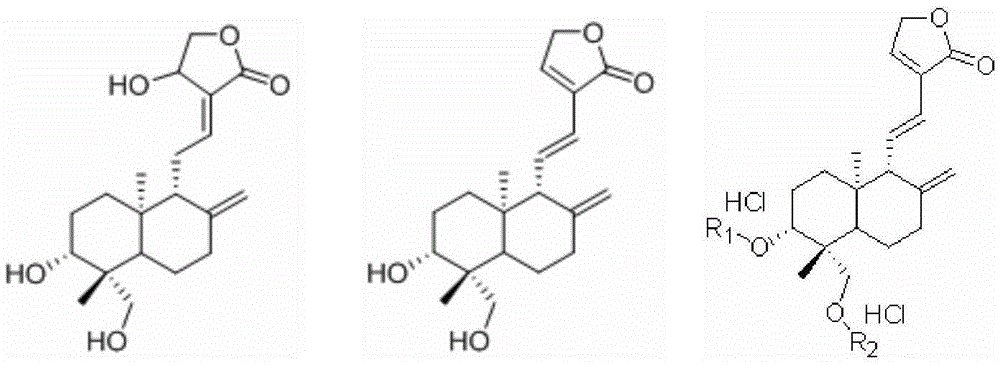
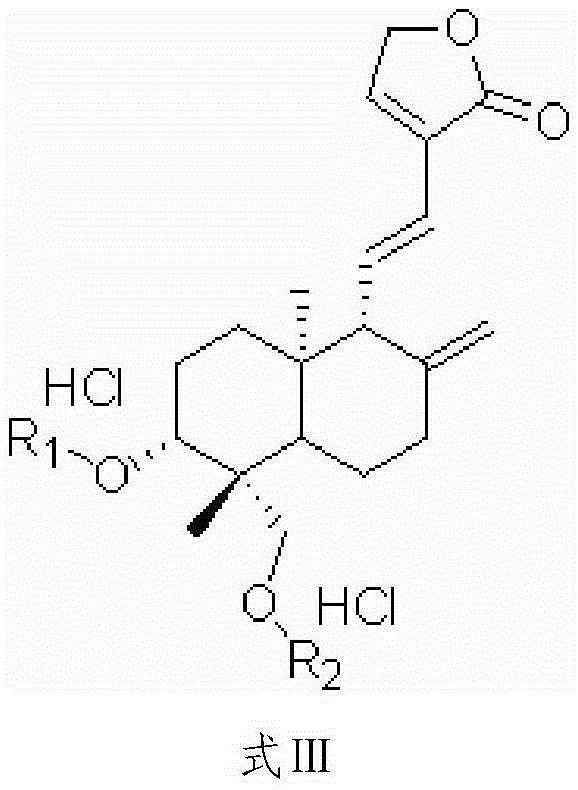
A kind of preparation method of dehydroandrographolide and its amino acid ester derivatives
A technology for dehydroandrographolide and andrographolide, which is applied in the field of medicine, can solve the problems of large demand for dehydroandrographolide, complicated separation process, and long time-consuming, so as to avoid adverse reactions, stable and controllable process, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: the preparation method of dehydroandrographolide
[0059] Get andrographolide 35.0g (0.1mol), add 60ml pyridine (equivalent to 1.71 times volume of andrographolide weight) and 0.5g aluminum oxide (equivalent to 1.43% of andrographolide weight), stir slowly and heat up to 120 ℃ , reflux reaction for 4 hours, filter after the reaction is completed, then add the filtrate to 200ml water (equivalent to 5.71 times the weight of andrographolide), add and stir synchronously, after adding completely, let it stand for 5 hours, filter, and shower with water for many times The pyridine was washed off, (when the pyridine was detected by HPLC was less than 0.1%), and dried to obtain 32.3 g of dehydroandrographolide, which was off-white. After recrystallization in 100 ml of 60% ethanol, 30.9 g of white needle crystals of dehydroandrographolide were obtained, with a yield of 93.1% and a purity of 99.5%.
Embodiment 2
[0060] Embodiment 2: the preparation method of dehydroandrographolide
[0061] Get andrographolide 35.0g (0.1mol), add 60ml pyridine (equivalent to 1.71 times volume of andrographolide weight) and 0.3g sodium sulfite (equivalent to 0.86% of andrographolide weight), stir slowly and heat up to 115 ℃, Reflux for 5 hours. Filter after completion of the reaction, slowly add the filtrate to 200ml of water (equivalent to 5.71 times the weight of andrographolide), add and stir synchronously, after adding completely, leave standstill for 5 hours, filter, rinse with water several times to remove pyridine, After drying, 32.5 g of dehydroandrographolide was obtained, and the product was off-white. After recrystallization in 100 ml of 70% ethanol, 30.6 g of white needle crystals of dehydroandrographolide were obtained, with a yield of 92.2% and a purity of 99.6%.
Embodiment 3
[0062] Embodiment 3: the preparation method of dehydroandrographolide
[0063] Get andrographolide 35.0g (0.1mol), add 60ml pyridine (equivalent to 1.71 times volume of andrographolide weight) and 0.5g aluminum oxide (equivalent to 1.43% of andrographolide weight) and 0.3g sodium sulfite (equivalent to andrographolide weight 1.43%) 0.86% of the lactone weight), stirred and slowly heated to 125° C., and refluxed for 4 hours. Filter after completion of the reaction, add the filtrate to 200ml of water (equivalent to 5.71 times the weight of andrographolide), add and stir simultaneously, after adding completely, let it stand for 5 hours, filter, rinse with water several times to remove pyridine, and dry , to obtain dehydroandrographolide 32.6g, and the product is off-white. After recrystallization in 100 ml of 60% ethanol, 30.6 g of white needle crystals of dehydroandrographolide were obtained, with a yield of 92.2% and a purity of 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com