Method for recovering ammonium chloride in hexamethyldisilazane preparation process
A technology for the preparation of hexamethyldisilazane, which is applied in chemical instruments and methods, ammonium halides, organic chemistry, etc., can solve problems such as low yield, high energy consumption, and reduced yield of the target product, and achieve steps Simple, no waste water discharge, reduced decomposition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
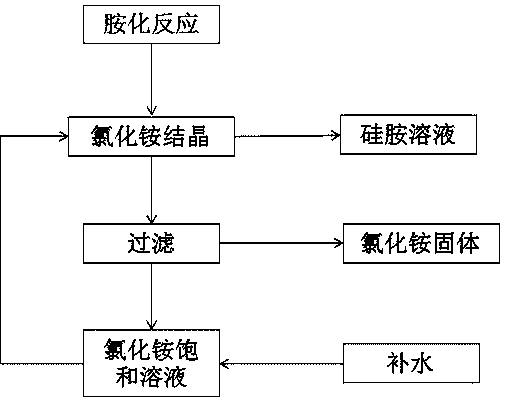
[0027] The recovery method of ammonium chloride in the preparation process of hexamethyldisilazane comprises the following steps:
[0028] (1) Take 400mL of the reaction solution after the amination reaction of trimethylsilyl chloride, add 400mL of a saturated solution of ammonium chloride, and stir for 10 minutes;
[0029] (2) Stand still for 5 minutes to separate the phases, and take out 273 mL of the organic phase of the toluene solution of hexamethyldisilazane;
[0030] (3) Add 400mL saturated ammonium chloride solution to the remaining mixture in step (2), and stir for 10 minutes;
[0031] (4) Stand still for 5 minutes to separate the phases, and take out 50 mL of the organic phase of the toluene solution of hexamethyldisilazane;
[0032] (5) Suction filter the remaining mixture in step (4) to obtain 161 g of ammonium chloride solid and 760 mL of mother liquor.
[0033] The above steps are carried out at 0-10°C.
Embodiment 2
[0035] The recovery method of ammonium chloride in the preparation process of hexamethyldisilazane comprises the following steps:
[0036] (1) Take 4L of the reaction solution after the amination reaction of trimethylchlorosilane, add 2L of saturated ammonium chloride solution, and stir to recrystallize the ammonium chloride in the solution;
[0037] (2) Stand still, separate the phases, and take out the organic phase of the toluene solution of hexamethyldisilazane in the uppermost layer;
[0038] (3) Add a saturated ammonium chloride solution of 0.5 times the volume of the mixed solution to the remaining mixed solution in step (2), and stir to recrystallize the ammonium chloride in the solution;
[0039] (4) Stand still, separate the phases, and take out the organic phase of the toluene solution of hexamethyldisilazane in the uppermost layer;
[0040] (5) Suction filter the remaining mixture in step (4) to obtain ammonium chloride solid and mother liquor.
[0041] The above...
Embodiment 3
[0043] The recovery method of ammonium chloride in the preparation process of hexamethyldisilazane comprises the following steps:
[0044] (1) Take 4L of the reaction solution after the amination reaction of trimethylchlorosilane, add 20L of a saturated solution of ammonium chloride, and stir to recrystallize the ammonium chloride in the solution;
[0045] (2) Stand still, separate the phases, and take out the organic phase of the toluene solution of hexamethyldisilazane in the uppermost layer;
[0046] (3) Add a saturated ammonium chloride solution 5 times the volume of the mixed solution to the remaining mixed solution in step (2), and stir to recrystallize the ammonium chloride in the solution;
[0047] (4) Stand still, separate the phases, and take out the organic phase of the toluene solution of hexamethyldisilazane in the uppermost layer;
[0048] (5) Suction filter the remaining mixture in step (4) to obtain ammonium chloride solid and mother liquor.
[0049] The above ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com