Humanized neutralizing antibody D5 against avian influenza virus (AIV) H7N9 as well as preparation method and application thereof
A bird flu virus and antibody technology, applied in antiviral agents, antiviral immunoglobulins, botanical equipment and methods, etc., can solve the problems of infection, time-consuming, and limitations of blood-borne diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
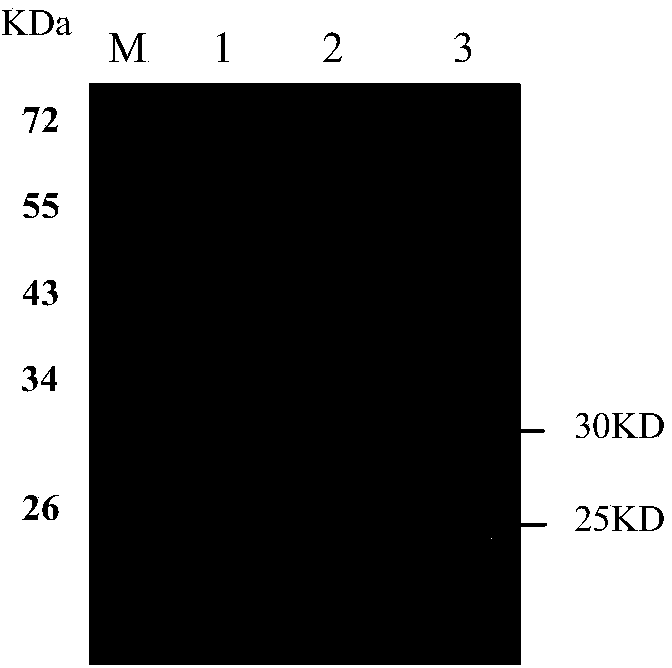
[0031] Example 1 Human anti-H7N9 avian influenza virus neutralizing antibody D5 and its preparation
[0032] 1 Materials and methods
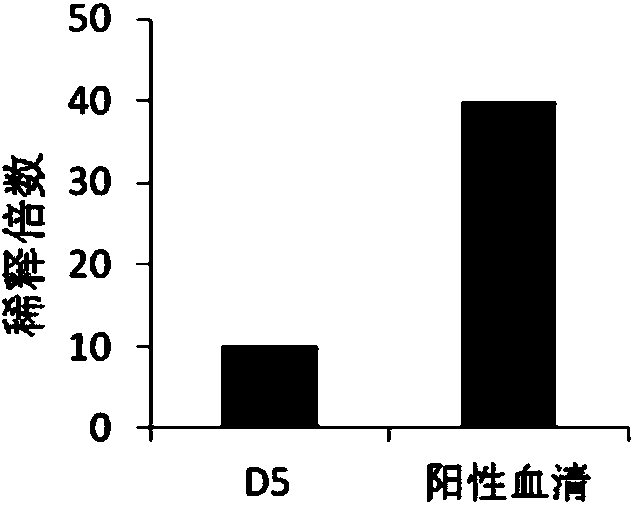
[0033]1.1 Source of virus, cell and vector: H7N9 virus A / Anhui / 1 / 2013 strain has been published in the literature (Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Li X, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Zhang Y, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y , Yuan Z, Shu Y.2013.Human infection with a novel avian-origin influenza A(H7N9)virus.N Engl J.Med.368:1888–1897), provided by the Institute of Pathogen Biology, Chinese Academy of Medical Sciences. The cells used for the in vitro neutralization experiment of H7N9 avian influenza virus were MDCK cells, which were purchased from ATCC. The bacterial strain used to construct the phage antibody library was XL1-Blue (Stratagene, U...
Embodiment 2
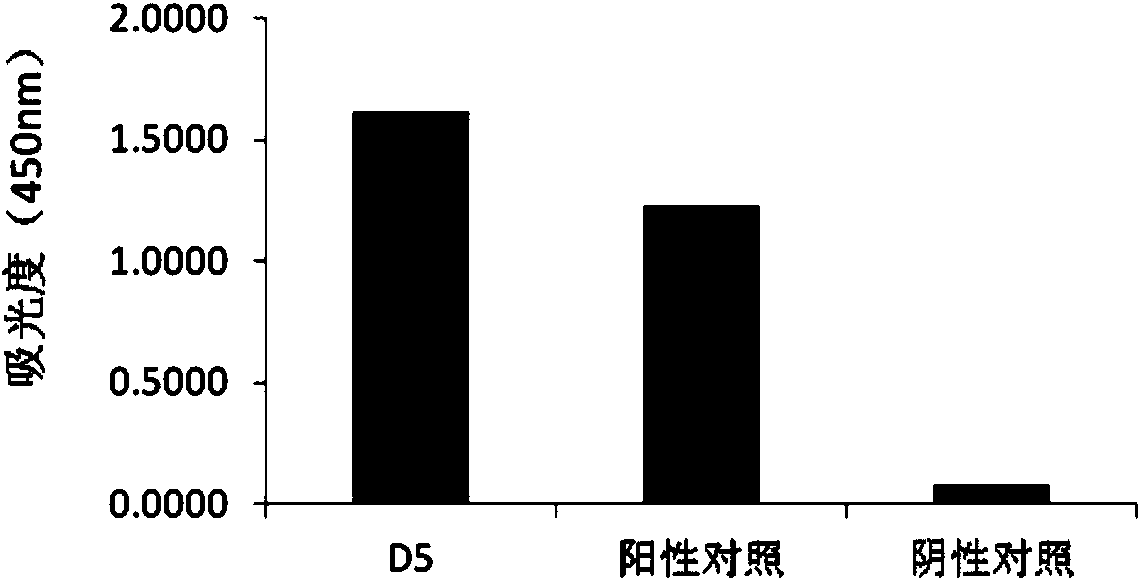
[0104] Example 2 Application of Human Anti-H7N9 Avian Influenza Virus Neutralizing Antibody D5
[0105] At present, there are no specific vaccines and drugs for the prevention and treatment of acute respiratory infectious diseases caused by the H7N9 virus. The immunoglobulins used clinically are often derived from the convalescent serum of patients infected with the virus, which limits their mass production. At the same time, because it comes from serum, it is prone to infection of blood-borne diseases. The invention provides a new approach for the treatment of acute respiratory infectious diseases caused by the H7N9 virus by using the human source anti-H7N9 avian influenza virus genetically engineered antibody D5 to replace the blood-derived immunoglobulin.
Embodiment 3
[0106] Example 3 Method for preparing full antibody immunoglobulin IgG by using neutralizing antibody D5
[0107] 1. Expression and purification of whole antibody IgG
[0108] ① Construction of whole antibody recombinant expression plasmid: first use primers (upstream primer 5'-CCCAAGCTTGTTGCTCTGGATCTCTGGTGCCTACGGGGACATCCAGATGACCCAGTCTCCATCCTCC-3', downstream primer 5'-CTAGTCTAGAATTAACACTCTCCCCTG-3') to amplify the light chain segment of the Fab antibody, digest with XbaI / HindIII, Cloned into PIgG vector (provided by Scripps Research Institute, USA) (Christoph Rader, Mikhail Popkov, John A.Neves, and Carlos F.Barbas III. Integrinαvβ3-targeted therapy for Kaposi.s sarcoma with an in vitro-evolved antibody. The FASEB Journal .(October18, 2002) 10.1096 / fj.02-0281fje.), the vector PIgG-L was obtained. Then use primers (upstream primer 5'-GAGGAGCTCACTCCAGGTGCAGCTGCAGGAGTCGGGCCCAGGAC-3', downstream primer 5'-GAGGGGCCCTTGGTGGAGGCTGAGGAGACGGT-3') to amplify the heavy chain segment of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com