Process for synthesizing ethyl caproate by yeast display lipase synthesis
A technology of ethyl hexanoate and lipase, applied in the field of preparation of ethyl hexanoate, can solve the problems of difficult separation, regeneration, recycling, high production cost, long reaction time, etc., and achieves good operational stability and reaction time. The effect of shortening and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Production of Pichia pastoris whole-cell enzyme preparation displaying Candida antarctica lipase B (calb for short) on the surface.
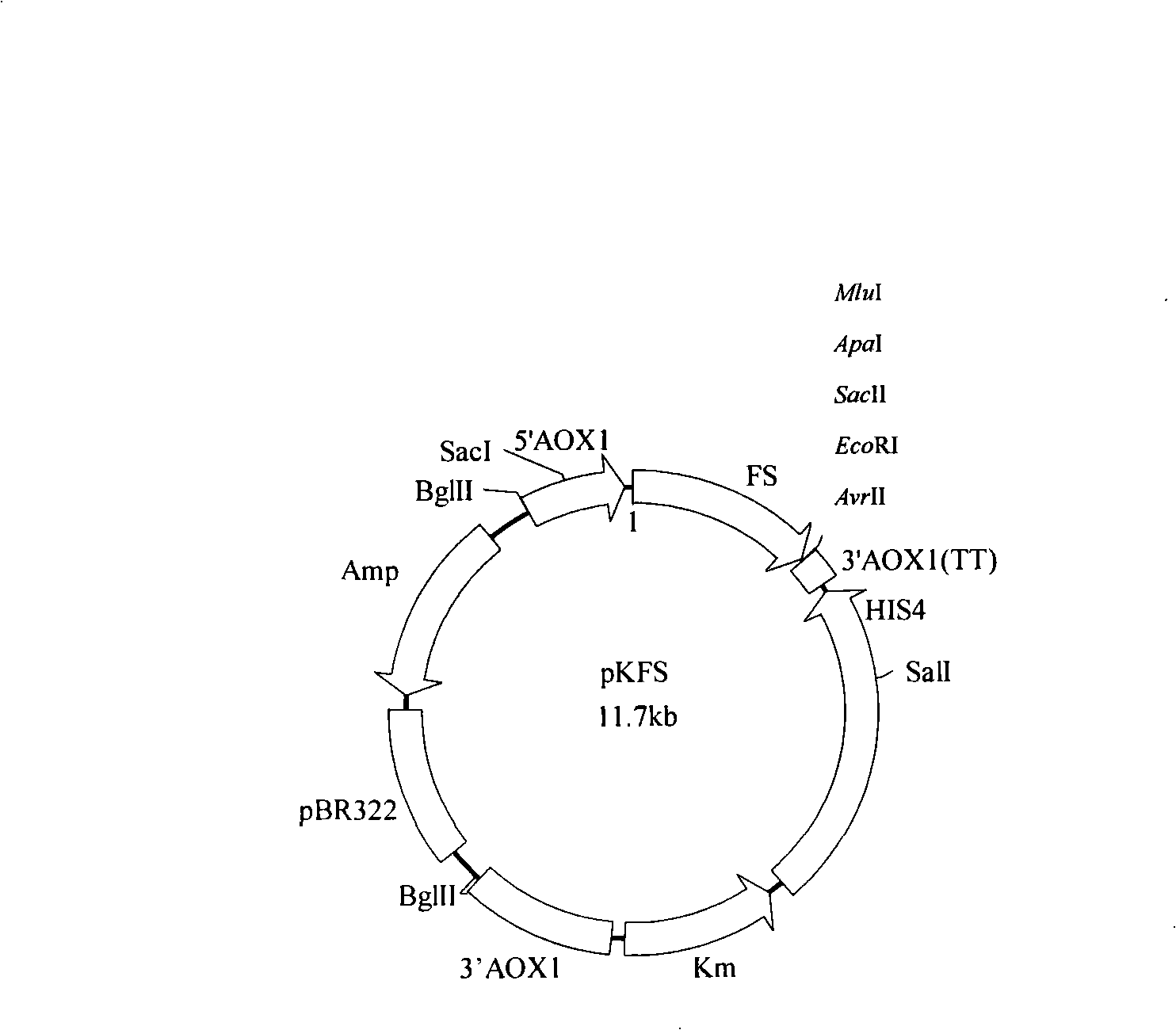
[0019] Firstly, primers were designed according to the nucleotide sequence of Candida antarctica lipase (calb) gene LF058, and full-length calb was amplified by PCR using Candida antarctica genomic DNA as a template. 30 cycles of 94°C for 1min, 60°C for 1min, and 72°C for 1min, and 72°C for 10min. 100 μL of the PCR amplification product was digested with Eco RI and Not I, and 20 μL of the plasmid pPIC9K-Flop1 was digested with Eco RI and Not I, and purified with T 4 DNA ligase ligation, ligation with pMD18-T, transformation of E.coli Top10, and screening of positive transformants.
[0020] The verified recombinant plasmid pKFS-CALB was linearized by Sac I and transformed into P. pastorisGS115 by electrotransfer. The transformed products were spread on a G418 gradient plate and cultured at 30°C for 2 days. Pick part of the transform...
Embodiment 2
[0023] (1) Production of Pichia pastoris whole-cell enzyme preparation displaying Rhizomucor miehei lipase (abbreviated as RML).
[0024] Primers were designed according to the Rhizomucor miehei lipase RML precursor sequence (P19515, GI: 417256) reported in GenBank, and the plasmid PMD18-T-RML was used as a template for PCR amplification. The PCR system is template 1 μL; 10×Taq DNA polymerase buffer 5 μL (containing Mg2+); 2.5 mmol / L dNTP 4 μL; 20 μM mol / L upstream and downstream primers 1 μL each; Taq DNA polymerase 0.75 μL, add sterile water to the total volume 50 μL. The reaction conditions are: pre-denaturation at 94°C for 5 min; denaturation at 94°C for 45 s, annealing at 45°C for 45 s, and extension at 72°C for 2 min, a total of 30 cycles; the 30th cycle was extended at 72°C for 10 min, and both the PCR product and the pKFS plasmid were treated with SnaBI and AvrII Double enzyme digestion, after constructing the recombinant plasmid pPIC9K-RML, use CaCl 2 The transforma...
Embodiment 3
[0028] (1) Production of Pichia pastoris whole-cell enzyme preparation displaying Rhizomucor miehei lipase (abbreviated as RML).
[0029] Primers were designed according to the Rhizomucor miehei lipase RML precursor sequence (P19515, GI: 417256) reported in GenBank, and the plasmid PMD18-T-RML was used as a template for PCR amplification. The PCR system is template 1 μL; 10×Taq DNA polymerase buffer 5 μL (containing Mg2+); 2.5 mmol / L dNTP 4 μL; 20 μM mol / L upstream and downstream primers 1 μL each; Taq DNA polymerase 0.75 μL, add sterile water to the total volume 50 μL. The reaction conditions were: pre-denaturation at 94°C for 5 min; denaturation at 94°C for 45 s, annealing at 45°C for 45 s, and extension at 72°C for 2 min, a total of 30 cycles; the 30th cycle was extended at 72°C for 10 min, and both the PCR product and the pKFS plasmid were double-coated with SnaBI and AvrII. Restriction digestion, after constructing the recombinant plasmid pPIC9K-RML, use the CaCl2 transf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com