Sulfothiazole-dithienopyrrole-benzodithiophene-containing copolymer and its preparation method and use
A technology containing thiothiazole and benzodithiophene, which is applied in chemical instruments and methods, tin organic compounds, semiconductor/solid-state device manufacturing, etc., can solve problems such as high cost, limited application, and complex production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
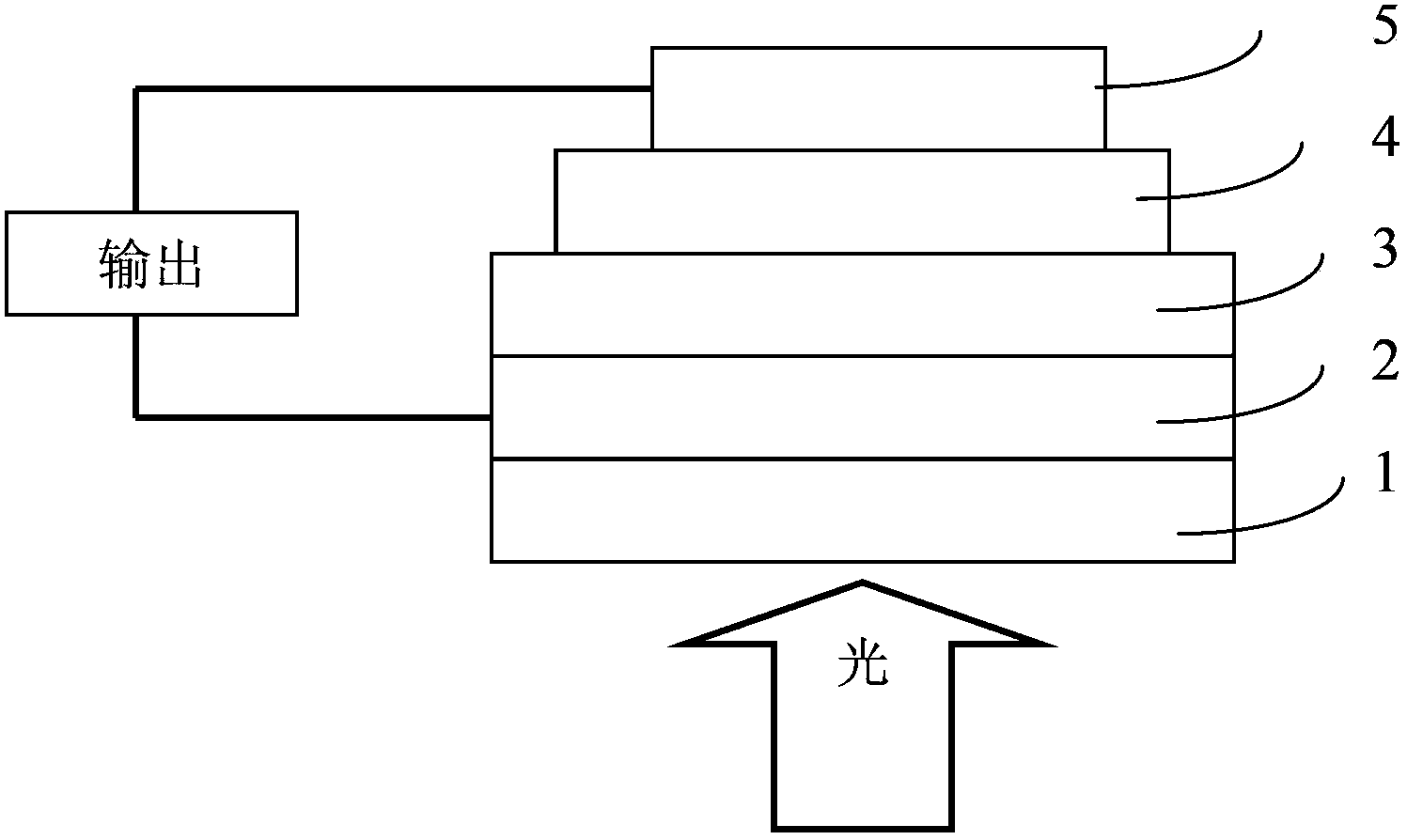
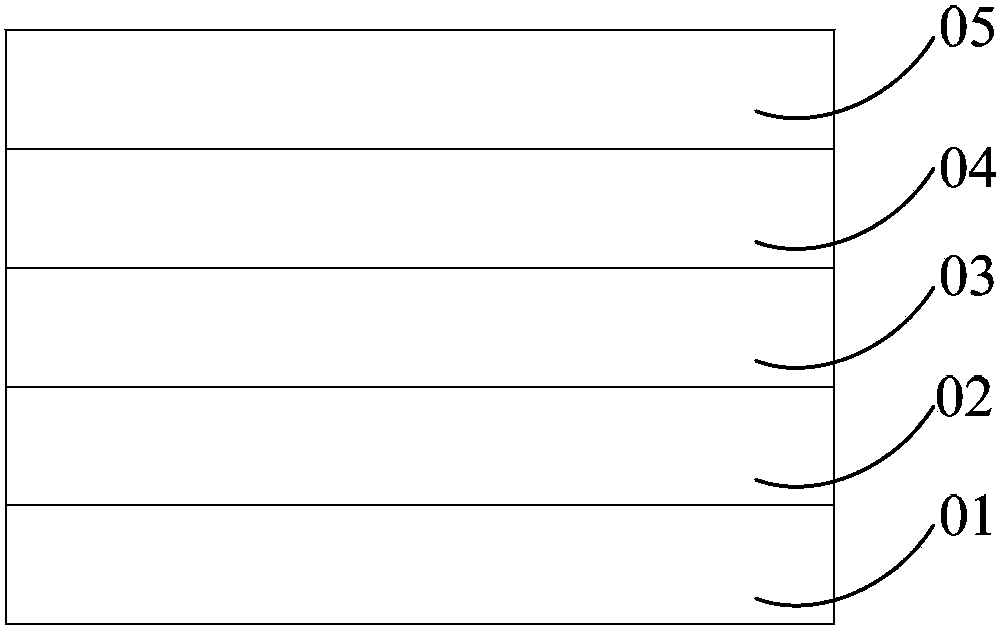
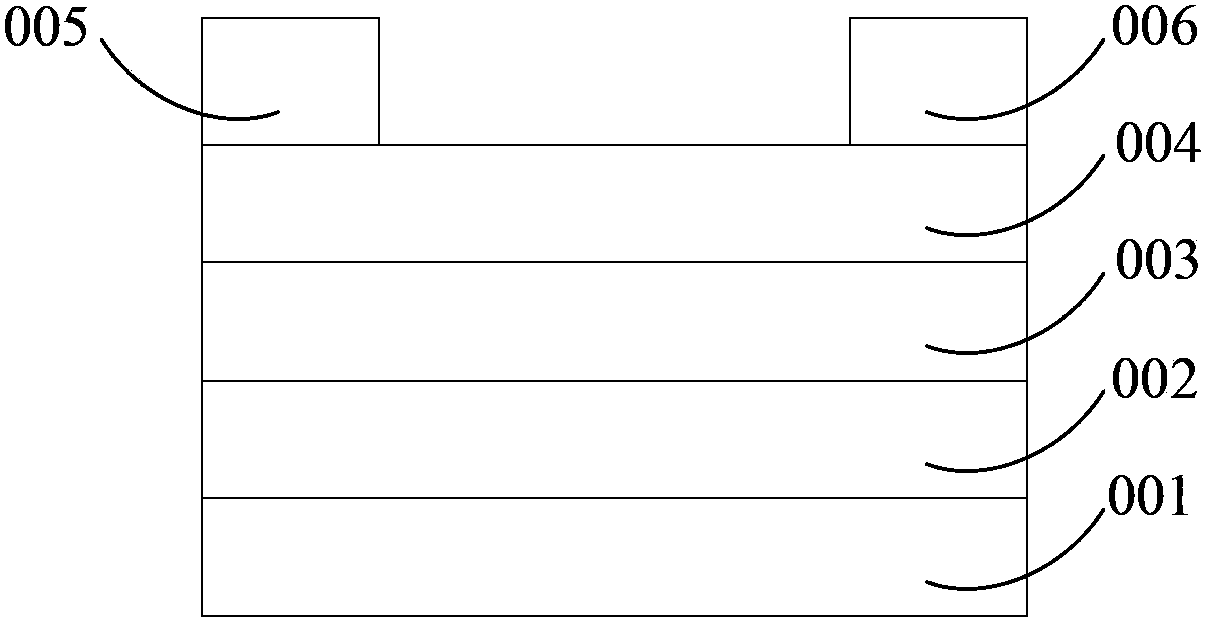
Image
Examples
Embodiment 1
[0062] A copolymer containing thiothiazole-dithienopyrrole-benzodithiophene, denoted as copolymer P1 (n=10), the structural formula is as follows:
[0063]
[0064] The preparation method comprises the following steps:
[0065] 1. Preparation of compound 2,6-bis(trimethyltin)-4,8-bis(4,5-dimethylmercapto-1,3-dithiol-2-aldehyde)benzo[1,2-b :4,5-b']dithiophene, denoted as A1;
[0066] (1) Preparation of compound 4,8-bis(4,5-dimethylmercapto-1,3-dithiol-2-aldehyde)benzo[1,2-b:4,5-b']dithiophene, Denote it as c1:
[0067] Provides compounds b1 and a, namely 2-dimethoxyphosphono-4,5-dimethylmercapto-1,3-dithiol and benzo[1,2-b:4,5-b']dithiophene- 4,8-diketone;
[0068] Under nitrogen protection, cool 1.22g (4.0mmol) b1 in 60mL of anhydrous THF to -78°C, slowly add 2.7mL of 1.5M LDA in cyclohexane (4mmol), and stir at -78°C for 3h , then add 0.44g (2.0mmol) a of 10mL anhydrous tetrahydrofuran solution to carry out Wittig-Horner reaction, keep warm for 0.5h and return to room...
Embodiment 2
[0083] A copolymer containing thiothiazole-dithienopyrrole-benzodithiophene, denoted as copolymer P2 (n=60), the structural formula is as follows:
[0084]
[0085] The preparation method comprises the following steps:
[0086] 1. Preparation of compound 2,6-bis(trimethyltin)-4,8-bis(4,5-dioctylmercapto-1,3-dithiol-2-aldehyde)benzo[1,2-b :4,5-b']dithiophene, denoted as A2;
[0087] (1) Preparation of compound 4,8-bis(4,5-dioctylmercapto-1,3-dithiol-2-aldehyde)benzo[1,2-b:4,5-b']dithiophene, Denote it as c2:
[0088] Provides compounds b2 and a, namely 2-dimethoxyphosphono-4,5-dioctylmercapto-1,3-dithiol and benzo[1,2-b:4,5-b']dithiophene- 4,8-diketone;
[0089] Under nitrogen protection, cool 2.2g (4.4mmol) of b2 in 60mL of anhydrous tetrahydrofuran to -78°C, slowly add 4.0mL of 1.5M LDA in cyclohexane (6mmol), and stir at -78°C for 3h , and then add 0.44g (2.0mmol) a 10mL anhydrous tetrahydrofuran solution to carry out Wittig-Horner reaction, keep warm for 0.5h and re...
Embodiment 3
[0102] A copolymer containing thiothiazole-bithienopyrrole-benzodithiophene, denoted as copolymer P3 (n=30), the structural formula is as follows:
[0103]
[0104] The preparation method comprises the following steps:
[0105] 1. Preparation of compound 2,6-bis(trimethyltin)-4,8-bis(4,5-bis(hexadecylmercapto)-1,3-dithiol-2-aldehyde)benzo[1, 2-b:4,5-b']dithiophene, denoted as A3;
[0106] (1) Preparation of compound 4,8-bis(4,5-bis(hexadecylmercapto)-1,3-dithiol-2-aldehyde)benzo[1,2-b:4,5-b'] Dithiophene, denoted as c3:
[0107] Provides compounds b3 and a, namely 2-dimethoxyphosphono-4,5-di(hexadecylmercapto)-1,3-dithiol and benzo[1,2-b:4,5-b'] Dithiophene-4,8-dione;
[0108] Under the protection of argon, cool the solution of 2.18g (3.0mmol) b3 in 60mL of anhydrous tetrahydrofuran to -78°C, slowly add 3.0mL of 1.5M LDA in cyclohexane (4.5mmol), and stir the reaction at -78°C After 2h, add 0.264g (1.2mmol) a of 15mL anhydrous tetrahydrofuran solution to carry out Witt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com