Synthesis method of aspoxicillin
A technology of apicillin and a synthesis method, applied in the field of chemical drug synthesis, can solve the problems of incomplete amino protection and removal, unsafe industrial production, reduced product yield and the like, and achieves overcoming poor reaction selectivity, less impurity residues, and by-products. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
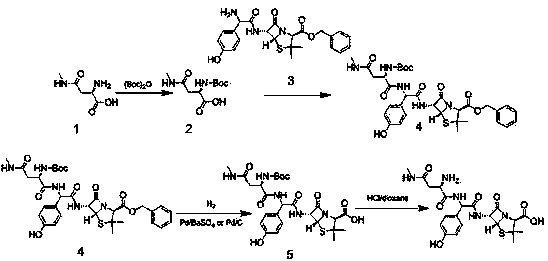
[0032] Add D-2-amino-3-methylaminopropionic acid (100g, 0.68mol) into the three-necked flask, then add DCM and stir to dissolve under ice-water bath, then add triethylamine (103g, 1.02mol) and dicarbonate Tert-butyl ester (224g, 1.02mol). When the temperature of the reaction system dropped to 5°C, 4-dimethylaminopyridine (5g, 0.04mol) was added and reacted at this temperature for 5h. Concentrate, pour the concentrated solution into a separatory funnel, add water (2000ml), adjust PH=2 with 1mol / L HCl, extract with ethyl acetate (2000ml), wash with water 3 times (2000ml×3), saturated saline (2000ml×3), dried over anhydrous sodium sulfate, distilled off ethyl acetate under reduced pressure, added an appropriate amount of petroleum ether, stirred and crystallized at 0°C, filtered with suction, washed with a small amount of petroleum ether, and dried in vacuum. Compound 2 (163.4 g, white solid, yield 97%) was obtained.
[0033] Add compound 2 (150g, 0.60mol) and compound 3 (260g, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com