Medicine for treating hyperacidity and preparation method thereof
A technology for hyperacidity and drugs, applied in drug combinations, active ingredients of aluminum/calcium/magnesium, digestive system, etc., can solve the problems of metronidazole with large side effects, unsuitable for long-term use, and unfavorable normal growth of children, etc. Mild effect of drug action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
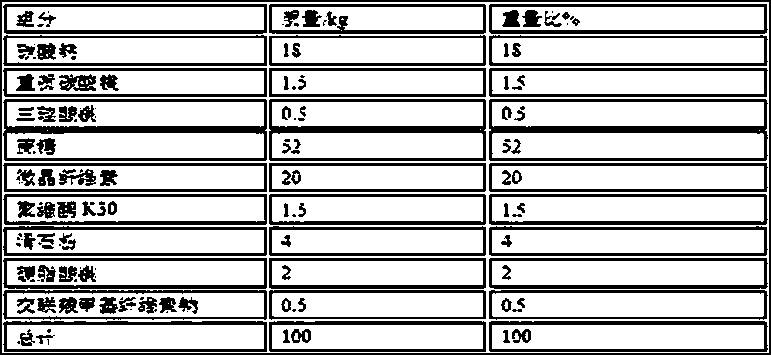
[0029] Weigh the raw and auxiliary materials of the following prescription quantities:
[0030]
[0031] Preparation:
[0032] (1) Grind each component in the formula into fine powders passing through an 80-mesh sieve; mix the formula amounts of calcium carbonate, heavy magnesium carbonate, magnesium trisilicate, sucrose, and microcrystalline cellulose evenly;
[0033] (2) Dissolve povidone in the prescribed amount in ethanol solution containing peppermint oil as a binder;
[0034] (3) Add the binder to the material obtained in step (1), mix evenly, pass through a 16-mesh sieve to granulate, boil and dry at 70°C for 5-45 minutes, and granulate with a 10-16 mesh sieve;
[0035] (4) Add talcum powder, magnesium stearate, and croscarmellose sodium in the prescribed amount to the premix prepared in step (3), and mix evenly to obtain drug granules for treating hyperacidity.
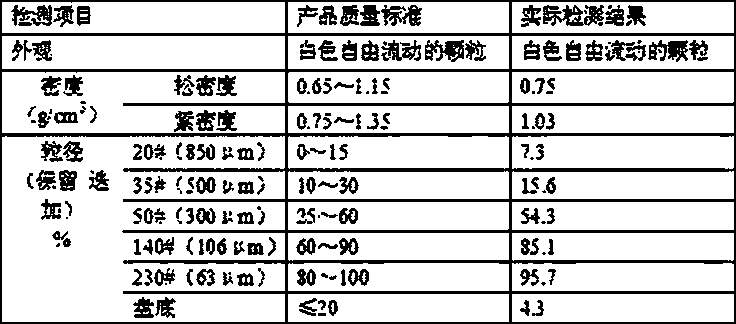
[0036] For the product quality standards and actual test results produced by the above-mentioned prepa...
Embodiment 2
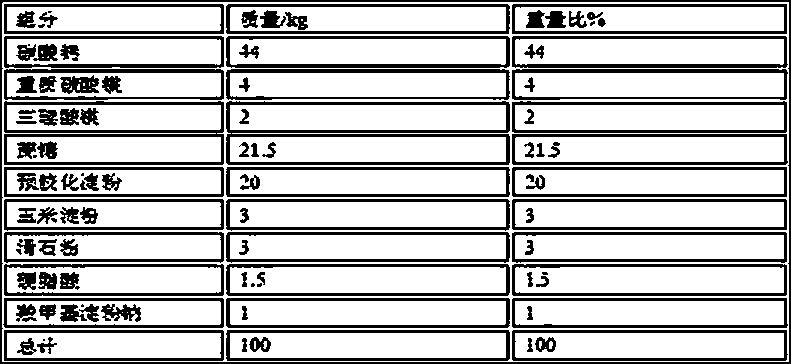
[0039] Weigh the raw and auxiliary materials of the following prescription quantities:
[0040]
[0041] Preparation:
[0042] (1) Grind each component in the formula into a fine powder passing through an 80-mesh sieve; mix the calcium carbonate, heavy magnesium carbonate, magnesium trisilicate, sucrose, and corn starch in the formula amount evenly;
[0043] (2) Dissolve povidone in the prescribed amount in ethanol solution containing peppermint oil as a binder;
[0044] (3) Add the binder to the material obtained in step (1), mix evenly, pass through a 16-mesh sieve to granulate, boil and dry at 70°C for 5-45 minutes, and granulate with a 10-16 mesh sieve;
[0045] (4) Add talcum powder, stearic acid, and sodium carboxymethyl starch in prescribed amounts to the premix prepared in step (3), and mix evenly to obtain drug granules for treating hyperacidity.
[0046] For the product quality standards and actual test results produced by the above-mentioned preparation process...
Embodiment 3
[0049] Weigh the raw and auxiliary materials of the following prescription quantities:
[0050]
[0051] Preparation:
[0052] (1) Grind each component in the formula into a fine powder passing through an 80-mesh sieve; mix the calcium carbonate, heavy magnesium carbonate, magnesium trisilicate, and sucrose in the formula amount evenly;
[0053] (2) Dissolve povidone in the prescribed amount in ethanol solution containing peppermint oil as a binder;
[0054] (3) Add the binder to the material obtained in step (1), mix evenly, pass through a 16-mesh sieve to granulate, boil and dry at 70°C for 5-45 minutes, and granulate with a 10-16 mesh sieve;
[0055] (4) Add talcum powder, magnesium stearate, and croscarmellose sodium in the prescribed amount to the premix prepared in step (3), and mix evenly to obtain drug granules for treating hyperacidity.
[0056] For the product quality standards and actual test results produced by the above-mentioned preparation process:
[0057] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com