Method for detection and quality control of medicine composition
A quality control method and detection method technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as unreasonable wavelength selection, large baseline fluctuations, and no preliminary investigation on the durability of analysis methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
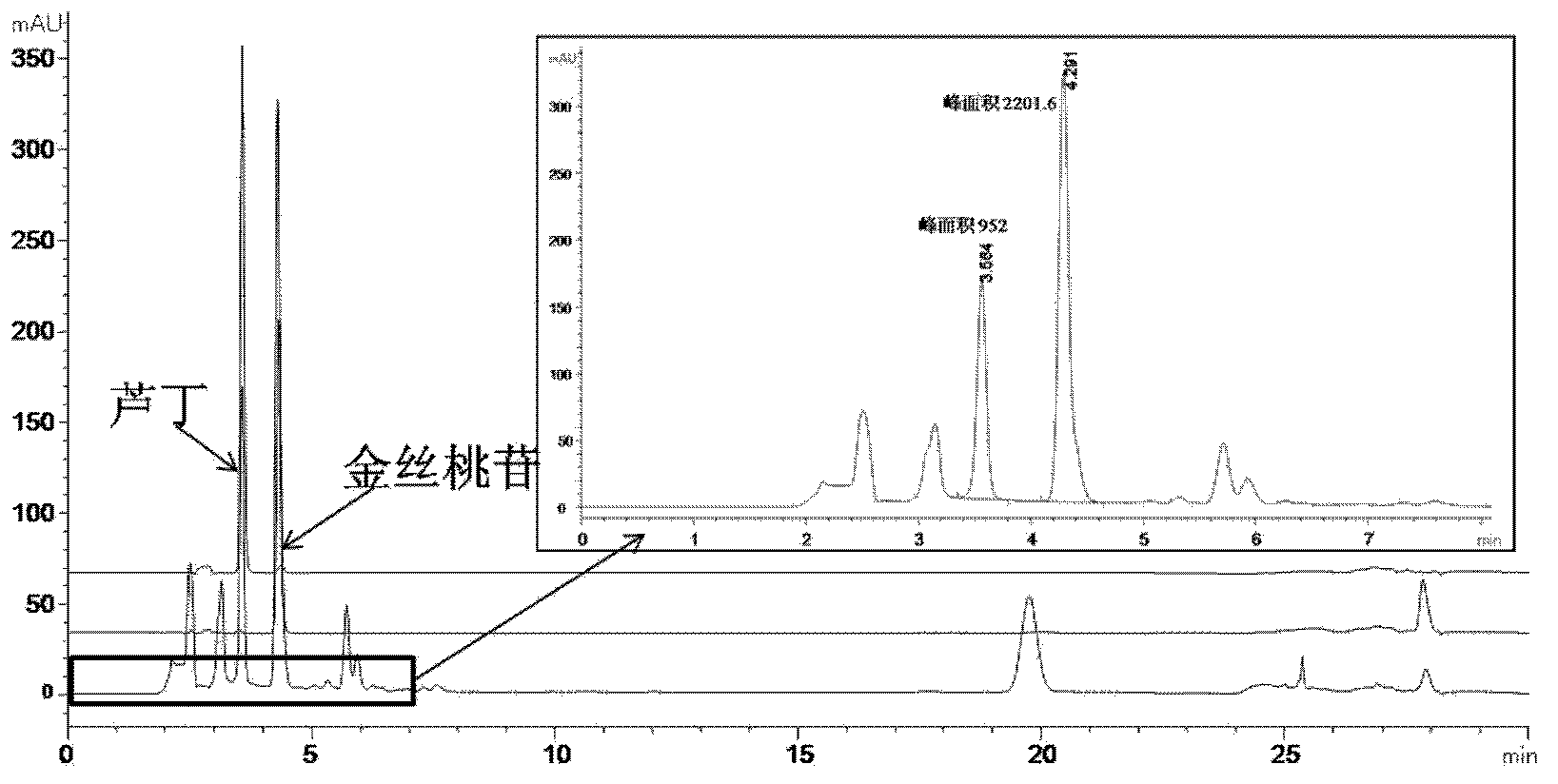
[0092] Embodiment 1 literature method (Fan Zhifeng, the content of hyperin, rutin and hypericin in different parts of Hypericum perforatum in Xinjiang and the influence of extraction process on them, Xinjiang Traditional Chinese Medicine, 2005,23,4:4-6 ) Determination of Hyperin and Rutin in Shugan Jieyu Capsules
[0093] 1. Sample
[0094] Shuganjieyu capsules (batch number: 111118) were provided by Chengdu Kanghong Pharmaceutical Group Co., Ltd.; hyperoside reference substance (111521-201004) was purchased from China Institute for the Control of Pharmaceutical and Biological Products; rutin reference substance (100080-200707) Purchased from China Institute for the Control of Pharmaceutical and Biological Products;
[0095] 2. Instruments and reagents
[0096] Agilent infinity1100 liquid chromatography (Agilent, USA); BS110S electronic balance (Sartorius, Germany); column Agilent Eclipse Plus C18, 5um, 250*4.6mm; acetonitrile (HPLC grade, Honeywell, LOT: AH015-4A); citric a...
Embodiment 2
[0101] Content detection of hyperin and rutin in the embodiment two Shuganjieyu capsules
[0102] 1. Sample
[0103] With embodiment one;
[0104] 2. Instruments and reagents
[0105] Agilent infinity1100 liquid chromatograph (Agilent, USA); Chromatographic column Agilent Eclipse Plus C18, 5um, 250*4.6mm; Methanol (HPLC, Honeywell, LOT: DF578); Acetonitrile (HPLC, Honeywell, LOT: AH015-4A); Absolute ethanol (Kelon, LOT: 20111105); formic acid (sigma-aldrich, LOT: BCBB7667); ultrapure water made by Milli-Q pure water system (Millipore Bedford, MA, USA)
[0106] 3. Detection method
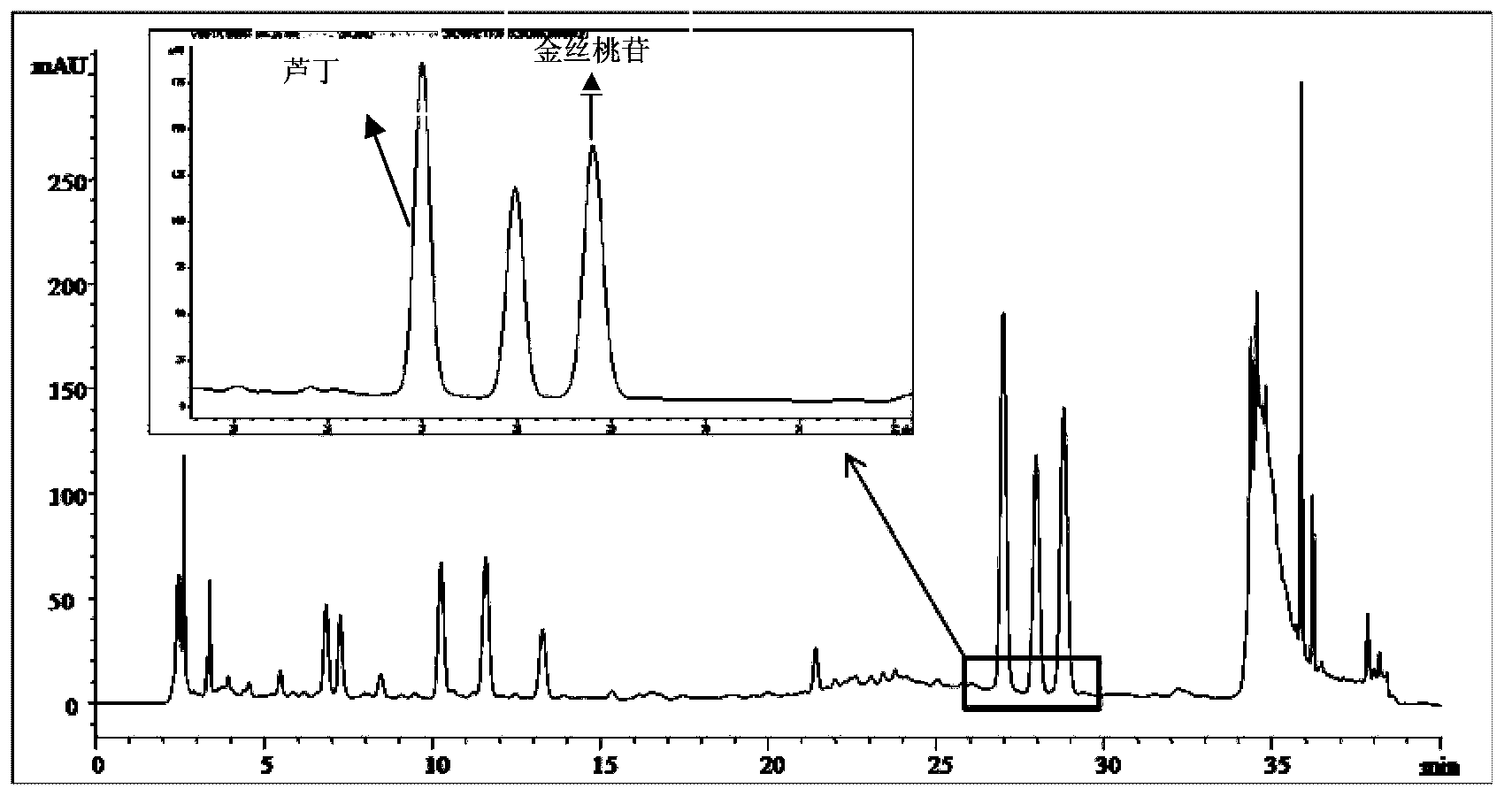
[0107] Preparation of the test solution: Take 0.2495 g of the content of Shugan Jieyu Capsules in a 50ml volumetric flask, add 50% ethanol to the mark, shake well, extract with ultrasound for 30 minutes, and filter with a 0.45 μm membrane to obtain the product.
[0108] High performance liquid chromatography detection conditions: high performance liquid chromatography detection conditions: mobil...
Embodiment 3
[0112] Content Detection of Hyperin and Rutin in Sanshugan Jieyu Capsules
[0113] 1. Drugs
[0114] With embodiment one;
[0115] 2. Instruments and reagents
[0116] Agilent infinity1100 liquid chromatograph (Agilent, USA); Chromatographic column Agilent Eclipse Plus C18, 5um, 250*4.6mm; Methanol (HPLC, Honeywell, LOT: DF578); Acetonitrile (HPLC, Honeywell, LOT: AH015-4A); Absolute ethanol (Kelon, LOT: 20111105); NaH 2 PO 4 .2H 2 O (Hua Da, LOT: 20101113); H 3 PO 4 (AR, Comeo, LOT: 20100906); Ultrapure water was made by Milli-Q pure water system (Millipore Bedford, MA, USA)
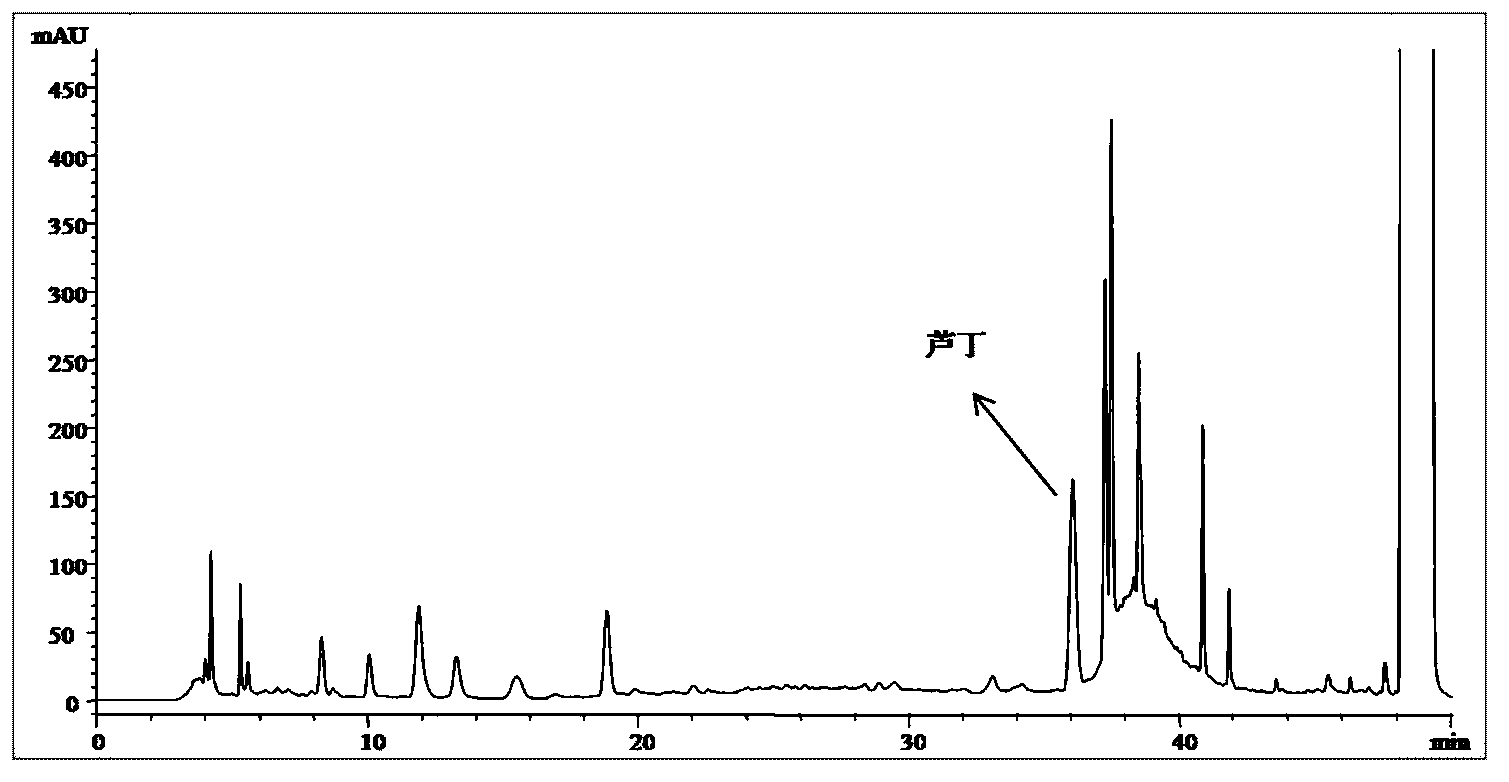
[0117] 3. Detection method
[0118] Preparation of the test solution: Take 0.2495 g of the content of Shugan Jieyu Capsules in a 50ml volumetric flask, add 50% ethanol to the mark, shake well, extract with ultrasound for 30 minutes, and filter with a 0.45 μm membrane to obtain the product.
[0119] High performance liquid chromatography detection conditions: mobile phase A: 50mM NaH 2 PO 4 Aq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com