Method for improving soluble expression of microbial transglutaminase in escherichia coli
A technology of transglutaminase and Escherichia coli, applied in the field of genetic engineering, can solve problems such as insolubility, and achieve the effects of improving soluble expression and obvious expression effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The construction method of embodiment 1 plasmid pET-MTG
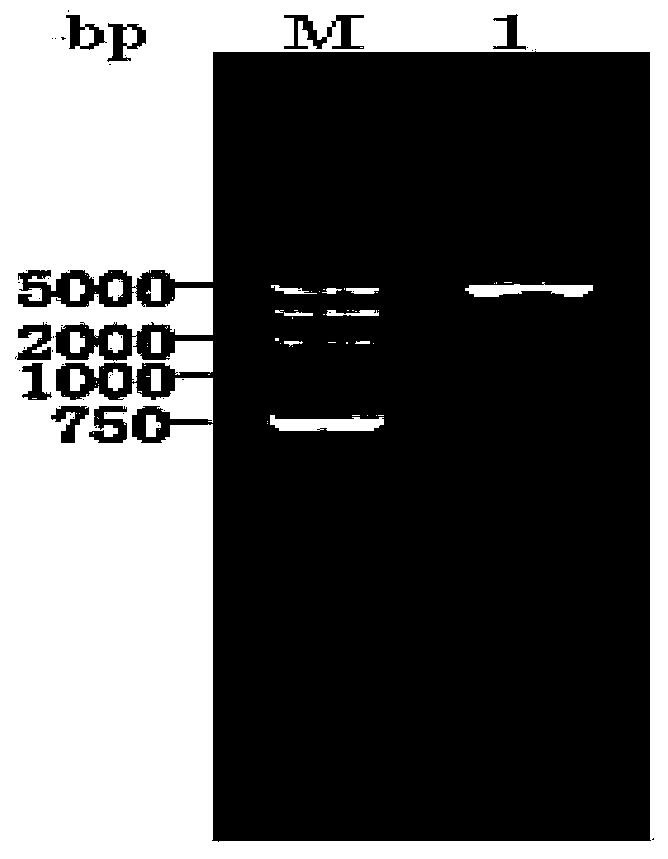
[0027] 1.1 PCR amplification of MTG gene
[0028] Using 1 μg of Streptoverticillium mobaraense genomic DNA as a PCR template, primer 1: 5′-TAAAAACATATGGACTCCGACGACAGGGTCAC-3′ and primer 2: 5′-TAAAAACTCGAGTTACGGCCAGCCCTGCTTTACC-3′ were designed for PCR amplification. The PCR reaction was carried out in a total volume of 50 μl, and the reaction conditions were: denaturation at 94°C for 5 min; denaturation at 94°C for 50 s, annealing at 58°C for 1 min, extension at 72°C for 2 min, and a total of 30 cycles; finally, extension at 72°C for 10 min.
[0029] 1.2 Construction of MTG expression plasmid
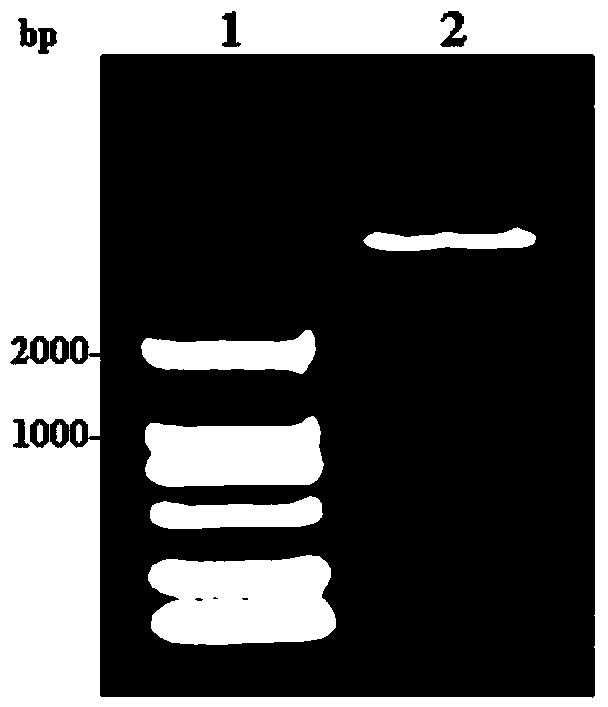
[0030] Gel recovery PCR amplified products were digested with NdeI and XhoI, and the digested fragments were recovered, ligated with the pET-22b vector that was also digested with NdeI and XhoI (ligated at 16°C for 16 hours), and transformed into E. coli DH5α competent cells , select positive clones, extract the plasmids...
Embodiment 2
[0031] The construction method of embodiment 2 plasmid pA-GESP
[0032] 2.1 Sticky-end PCR technique to amplify artificial operons containing molecular chaperones
[0033] According to the sequence of the upstream and downstream genes of the amplified artificial operon (GenBank ID: NC_010473.1), two pairs of primers were designed, primer 3: 5′-CATGGCAGCTAAAGACGTAAAATTC-3′ and primer 4: 5′-GTTAAGCTTTTGCTTTCGCTACAGT-3′, and primers 5: 5′-GCAGCTAAAGACGTAAAATTCGGTA-3′ and primer 6: 5′-TCGAGTTAAGCTTTTGCTTTCGCTA-3′, use primers 3 and 4, primers 5 and 6, respectively, perform PCR amplification in two tubes simultaneously, add 3 μl of plasmid E. coli Genomic DNA was used as a template. The PCR reaction was carried out in a total volume of 50 μl, and the reaction conditions were: denaturation at 94°C for 30 s; annealing at 55°C for 30 s, and extension at 72°C for 4 min, a total of 30 cycles.
[0034] Recover the PCR amplification product, measure the A260 value, mix the two PCR produc...
Embodiment 3
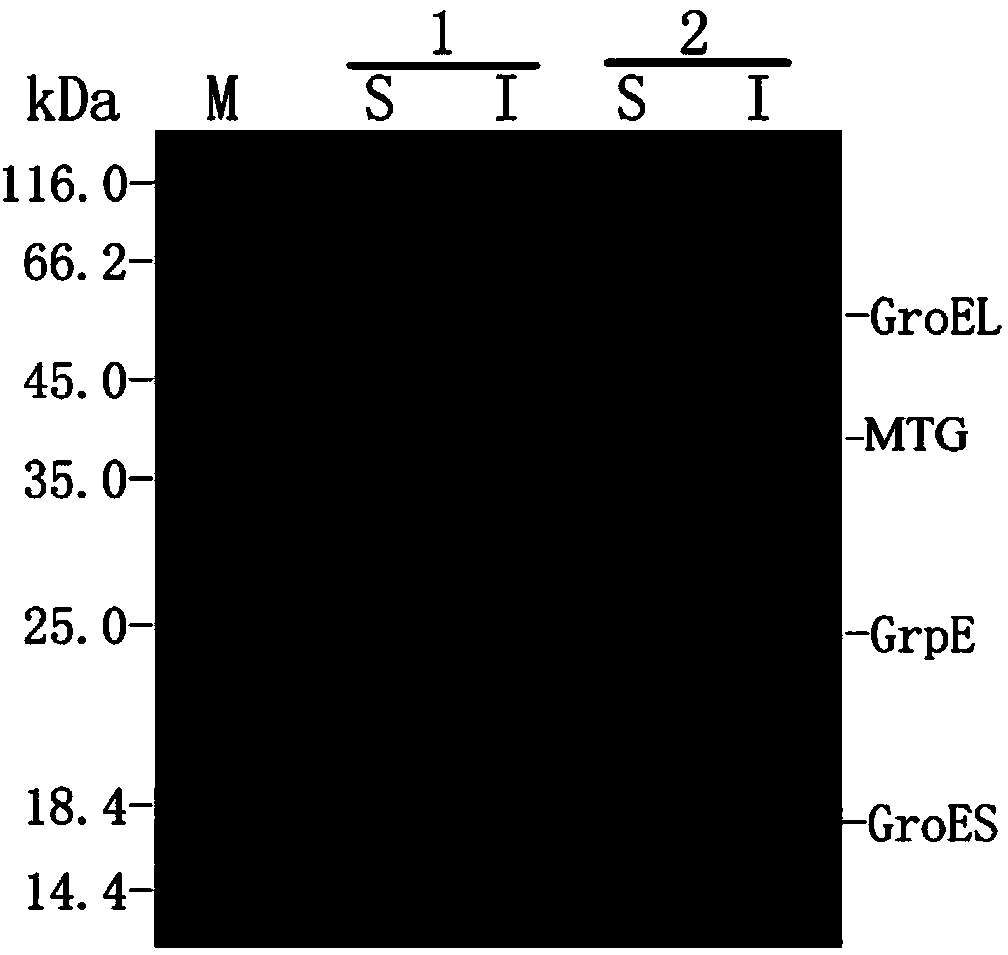
[0037] Co-transformation of embodiment 3 molecular chaperone and MTG
[0038] Co-transform Escherichia coli (Rosetta DE3) with pET-MTG and pA-GESP plasmids, and use the co-transformation of pET-MTG and pACYC plasmids as a control. The co-transformed bacterial solution contains 25 μg / ml ampicillin and 25 μg / ml chloramphenicol Plates resistant to two kinds of antibiotics were cultured upside down in a 37°C incubator for 12 hours. Pick a single colony and inoculate it in 10ml of LB liquid medium containing two antibiotics, 25μg / ml ampicillin and 25μg / ml chloramphenicol, culture overnight at 37°C with shaking at 220r / min; turn at 1:100 Into 100ml of LB medium, when OD 600 At about 0.6, add IPTG with a final concentration of 0.4mmol / L, induce at 28°C for 12h, centrifuge at 5000×g for 10min to collect the bacteria, add about 1ml of lysis buffer (100mmol / L sodium phosphate buffer, containing 300mmol / L NaCl and 10mmol / L imidazole, pH 8.0) sonicated at 300W power, 3s each time, 9s i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com