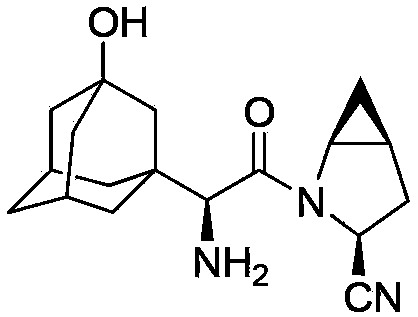
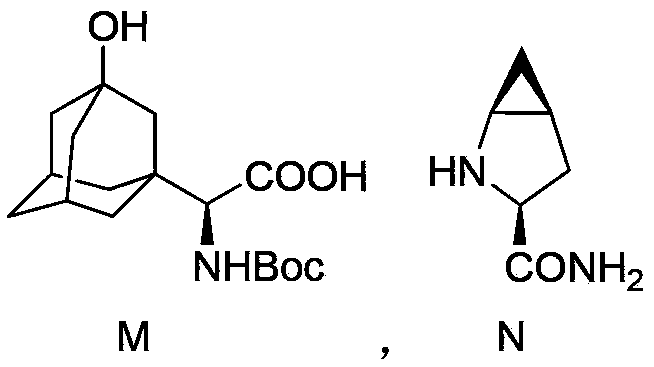
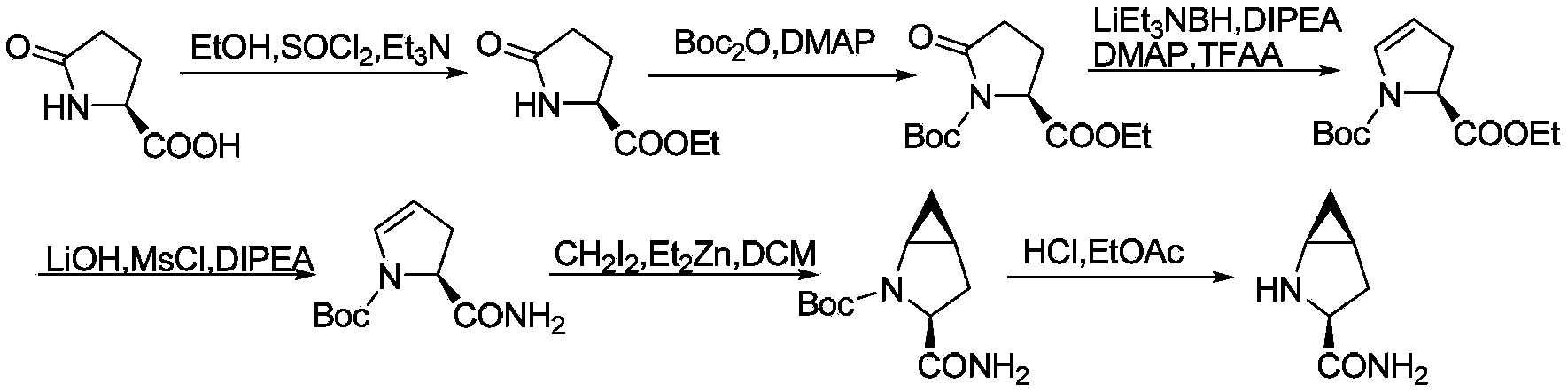
Preparation method of saxagliptin intermediate
A technology of compound and hydroxyl protecting group, which is applied in the field of preparation of saxagliptin intermediates, can solve the problems of harsh conditions, strong corrosion, and low reaction yield, and achieve low price, mild reaction conditions, and high reaction yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: S-2-benzylamino-pent-4-en-1-ol
[0043] S-2-amino-pent-4-en-1-ol (5.16g, 51.0mmol), benzaldehyde (6.0g, 56.6mmol) and 30mL methanol were placed in a 100ml two-necked round bottom flask, and the reactant was heated to The reaction was carried out at 40° C. for 12 hours. The reaction mixture was cooled with an ice bath and sodium borohydride (2.65 g, 70.0 mmol) was slowly added, and the reaction was continued for 3 hours after the addition. After the reaction was completed, 10 mL of water was added to quench the reaction, the solvent was removed under reduced pressure, and 30 mL of ethyl acetate was added, washed with water and dried to obtain 9.48 g of S-2-benzylamino-pent-4-en-1-ol with a yield of 97.3%. .
Embodiment 2
[0044] Example 2: S-2-benzylamino-2-formyl-pent-4-en-1-ol
[0045] The substrate S-2-benzylamino-pent-4-en-1-ol (3.0 g, 15.7 mmol) and 30 mL of ethyl formate were placed in a 50 mL single-necked round-bottomed flask, and the reactants were heated to reflux for 24 hours. After the reaction is completed, the solvent is removed to obtain 3.5 g of crude S-2-benzylamino 2-formyl-pent-4-en-1-ol, which can be directly carried out to the next step of the reaction.
Embodiment 3
[0046] Example 3: S-2-benzylamino-2-formyl-1-tert-butyldimethyloxy-pent-4-ene
[0047] The crude product S-2-benzylamino 2-formyl-pent-4-en-1-ol (3.0 g, 13.7 mmol), imidazole (1.87 g, 27.4 mmol) and 20 mL of dichloromethane were placed in 100 mL of two round bottoms In the flask, tert-butyldimethylsilyl chloride (3.1 g, 20.6 mmol) was dissolved in 10 mL of dichloromethane and slowly added dropwise. After the dropwise addition, the reaction was performed for 3 hours, and the reaction end point was determined by thin layer chromatography. After the reaction was completed, 15 mL of water was added to quench and stirred for 0.5 hours. The organic phase was separated, washed with water and dried. After removing the solvent, crude product S-2-benzylamino 2-formyl-1-tert-butyldimethyloxy-pentane was obtained. -4-ene 6g, the crude product was purified by column chromatography to obtain product 4.5g, yield 98.7%.
[0048] 1 HNMR (CDCl 3 ,400MHz)δ(ppm):δ-0.014-0.007(m,6H),2.27-2.38(m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com