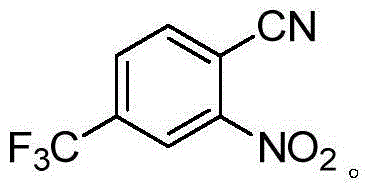
Preparation method for 2-nitro-4-trifluoromethyl cyanobenzene
A technology of trifluoromethylbenzonitrile and halotrifluorotoluene, applied in the field of preparation of 2-nitro-4-trifluoromethylbenzonitrile, can solve the problems of unsuitable large-scale industrial production, sodium cyanide and Potassium cyanide is highly toxic and potassium ferrocyanide has poor solubility, achieving the effect of low price, low toxicity and not easy to be poisoned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
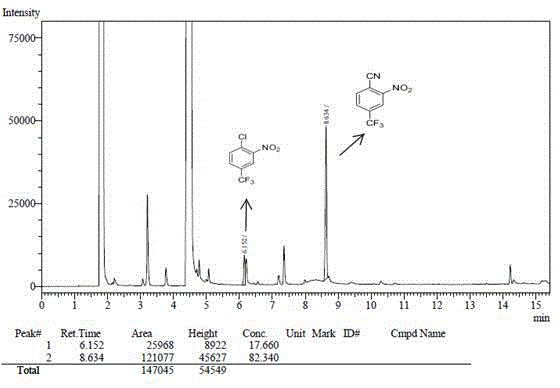
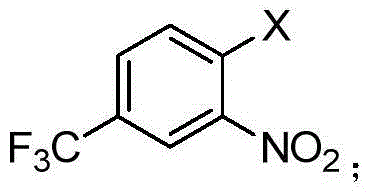
[0034] Add 1mmol of cuprous iodide, 1mmol of 3-nitro-4-chlorobenzotrifluoride, 1.5mmol of phenylacetonitrile, 2ml of N,N-dimethylformamide into a 10ml reaction flask, stir and heat to 160°C under air, after 18 hours Stop responding. 50 microliters of the reaction solution was extracted with water and dichloromethane, and the organic phase was taken for gas chromatography analysis. The result showed that the reaction yield was 53%.
Embodiment 2
[0036] Add 1mmol of cuprous bromide, 1mmol of 3-nitro-4-chlorobenzotrifluoride, 1.5mmol of phenylacetonitrile, 2ml of N,N-dimethylformamide into a 10ml reaction flask, stir and heat to 160°C under air, after 18 hours Stop responding. 50 microliters of the reaction solution was extracted with water and dichloromethane, and the organic phase was taken for gas chromatography analysis. The result showed that the reaction yield was 38%.
Embodiment 3
[0038] Add 1mmol cuprous iodide, 1mmol 3-nitro-4-chlorobenzotrifluoride, 1.5mmol phenylacetonitrile, 2mN-methylpyrrolidone into a 10ml reaction flask, stir and heat to 160°C under air, and stop the reaction after 18 hours. 50 microliters of the reaction solution was extracted with water and dichloromethane, and the organic phase was taken for gas chromatography analysis. The result showed that the reaction yield was 67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com