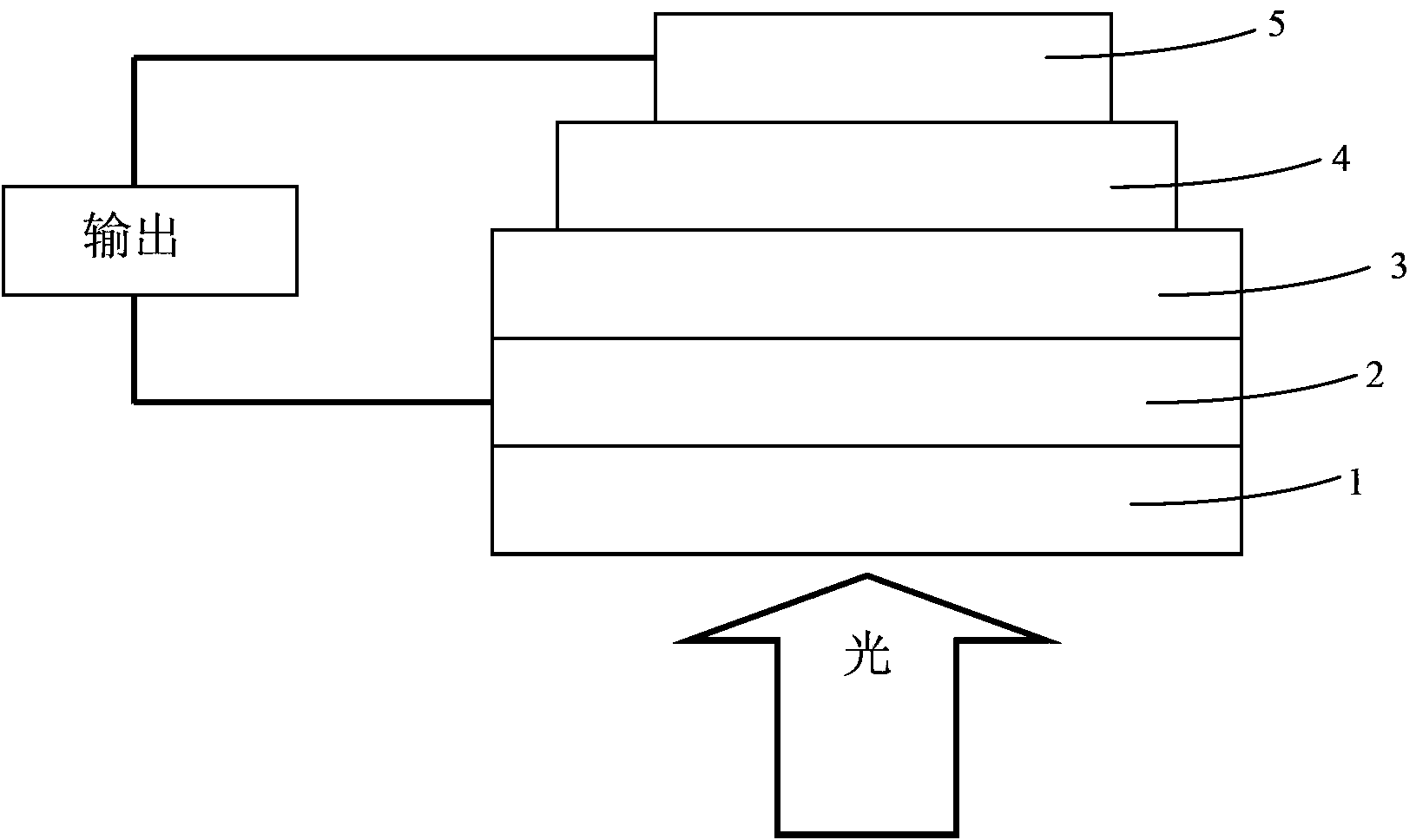
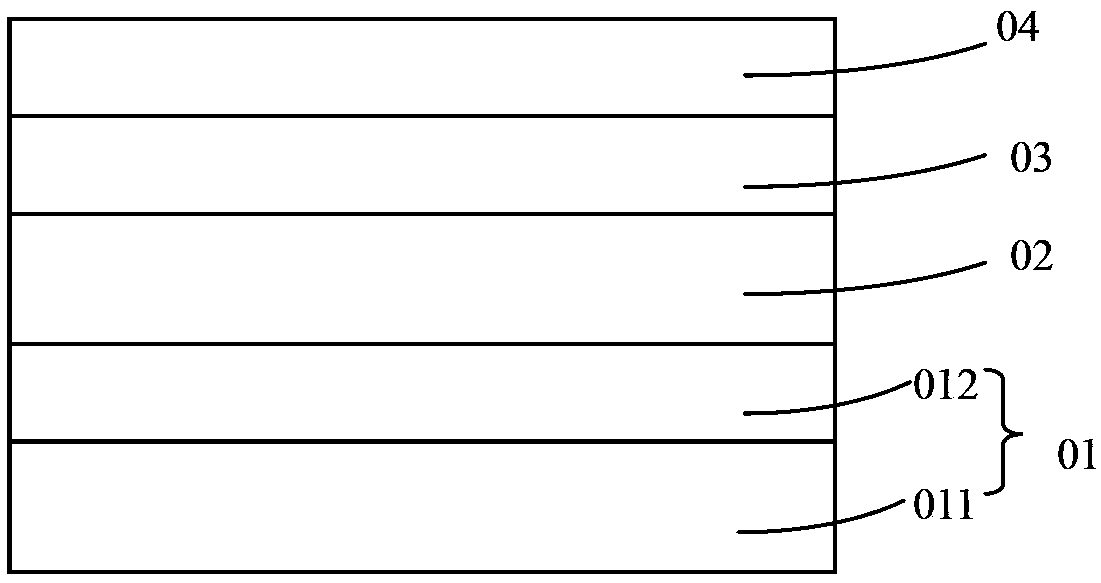
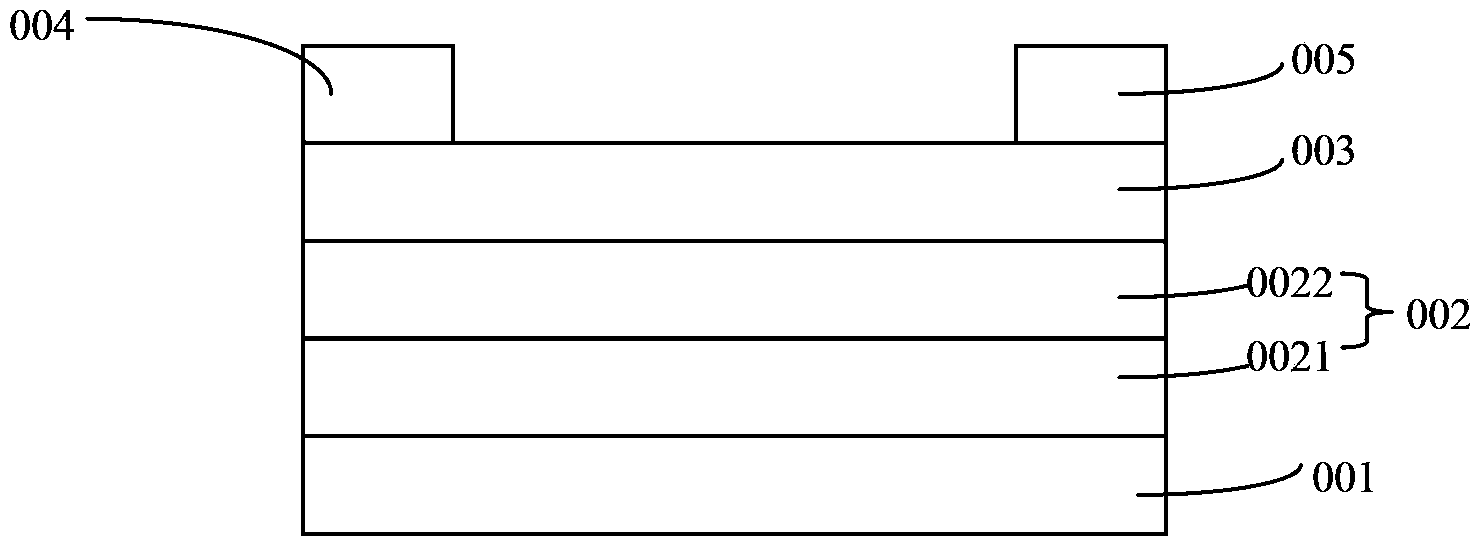
Conjugated polymer material containing pyrrole indolodithiapentalene-dithieno benzothiadiazole and preparation method and application thereof
A technology of benzothiadiazole and conjugated polymers, which is applied in the field of photoelectric materials and can solve the problems of low photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] This example provides a conjugated polymer containing pyrrole indolodithiophene-dithienobenzothiadiazole, the chemical structure of which is shown in P1 (n=5):
[0062]
[0063] The preparation steps of the above P1 are as follows:
[0064] Step 1, preparation of compound A1
[0065] S10. Synthesis of compound E (2,2'-(2,5-dibromo-1,4-phenyl)bis(3-bromothiophene))
[0066] Compound C (2,5-dibromo-1,4-diiodobenzene) and compound D (3-bromo-2-trimethyltinthiophene) represented by the following structural formulas are provided:
[0067]
[0068] Under nitrogen protection, lithium chloride (2.54g, 60mmol), compound C (4.8g, 10mmol), Pd(PPh 3 ) 4 (287.5mg, 0.25mmol), cuprous chloride (5g, 50mmol), compound D (3.59g, 11mmol) and tetrahydrofuran (60mL), the reactant was stirred at room temperature for 1 hour, then heated to 50°C for 3 hours; Cool to room temperature, filter the solid generated by the reaction to obtain the crude product of Compound E, yield 40%, MS (...
Embodiment 2
[0098] This example provides a conjugated polymer containing pyrrole indolodithiophene-dithienobenzothiadiazole, the chemical structure of which is shown in P2 (n=60):
[0099]
[0100] The preparation steps of above-mentioned P2 are as follows:
[0101] Step 1, preparation of compound A2
[0102] S10. Synthesis of compound E (2,2'-(2,5-dibromo-1,4-phenyl)bis(3-bromothiophene))
[0103] Provide compound C (2,5-dibromo-1,4-diiodobenzene) and compound D (3-bromo-2-trimethyltinthiophene), see Example 1 for the structural formula;
[0104] Under nitrogen protection, lithium chloride (2.63g, 62mmol), compound C (4.8g, 10mmol), Pd(PPh 3 ) 2 Cl 2 (0.7mg, 0.001mmol), cuprous chloride (5.15g, 52mmol), compound D (4.89g, 15mmol) and DMF (60mL), after the reaction was stirred at room temperature for 2 hours, it was heated to 55 ° C for 4 hours ; Cool to room temperature, filter the solid generated by the reaction to obtain the crude product of Compound E, with a yield of 39%, MS ...
Embodiment 3
[0128] This example provides a conjugated polymer containing pyrrole indolodithiophene-dithienobenzothiadiazole, the chemical structure of which is shown in P3 (n=30):
[0129]
[0130] The preparation steps of above-mentioned P3 are as follows:
[0131] Step 1, preparation of compound A3
[0132] S10. Synthesis of compound E (2,2'-(2,5-dibromo-1,4-phenyl)bis(3-bromothiophene))
[0133] Provide compound C (2,5-dibromo-1,4-diiodobenzene) and compound D (3-bromo-2-trimethyltinthiophene), see Example 1 for the structural formula;
[0134] Under the protection of nitrogen, lithium chloride (2.59g, 61mmol), compound C (4.8g, 10mmol), Pd(PPh 3 ) 4 (578mg, 0.5mmol), cuprous chloride (5.05g, 51mmol), compound D (3.91g, 12mmol) and toluene (60mL), the reactants were stirred at room temperature for 1.5 hours, then heated to 52°C for 3.5 hours; Cool to room temperature, filter the solid generated by the reaction to obtain the crude product of Compound E, the yield is 42%, MS (EI) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet resistance | aaaaa | aaaaa |
| Short circuit current density | aaaaa | aaaaa |
| Maximum brightness efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com