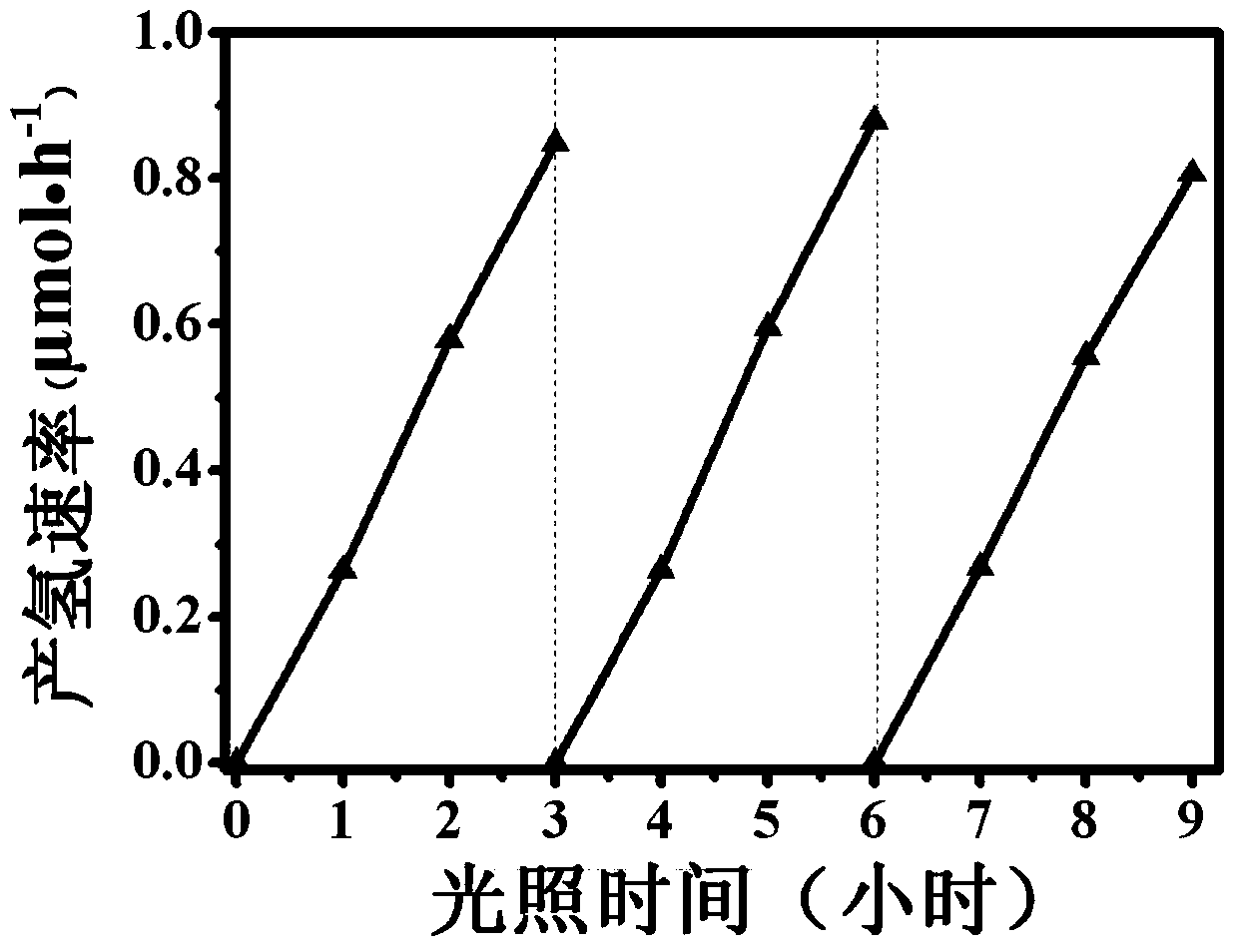
Patents
Literature
31results about How to "Improve photocatalytic hydrogen production efficiency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nickel single-active site graphite-phase carbon nitride-based photocatalytic material as well as preparation method and application thereof
InactiveCN109420514AReduce manufacturing costImproved response to visible lightPhysical/chemical process catalystsHydrogen productionNickelAqueous solution
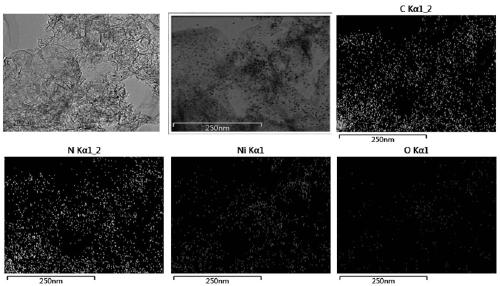
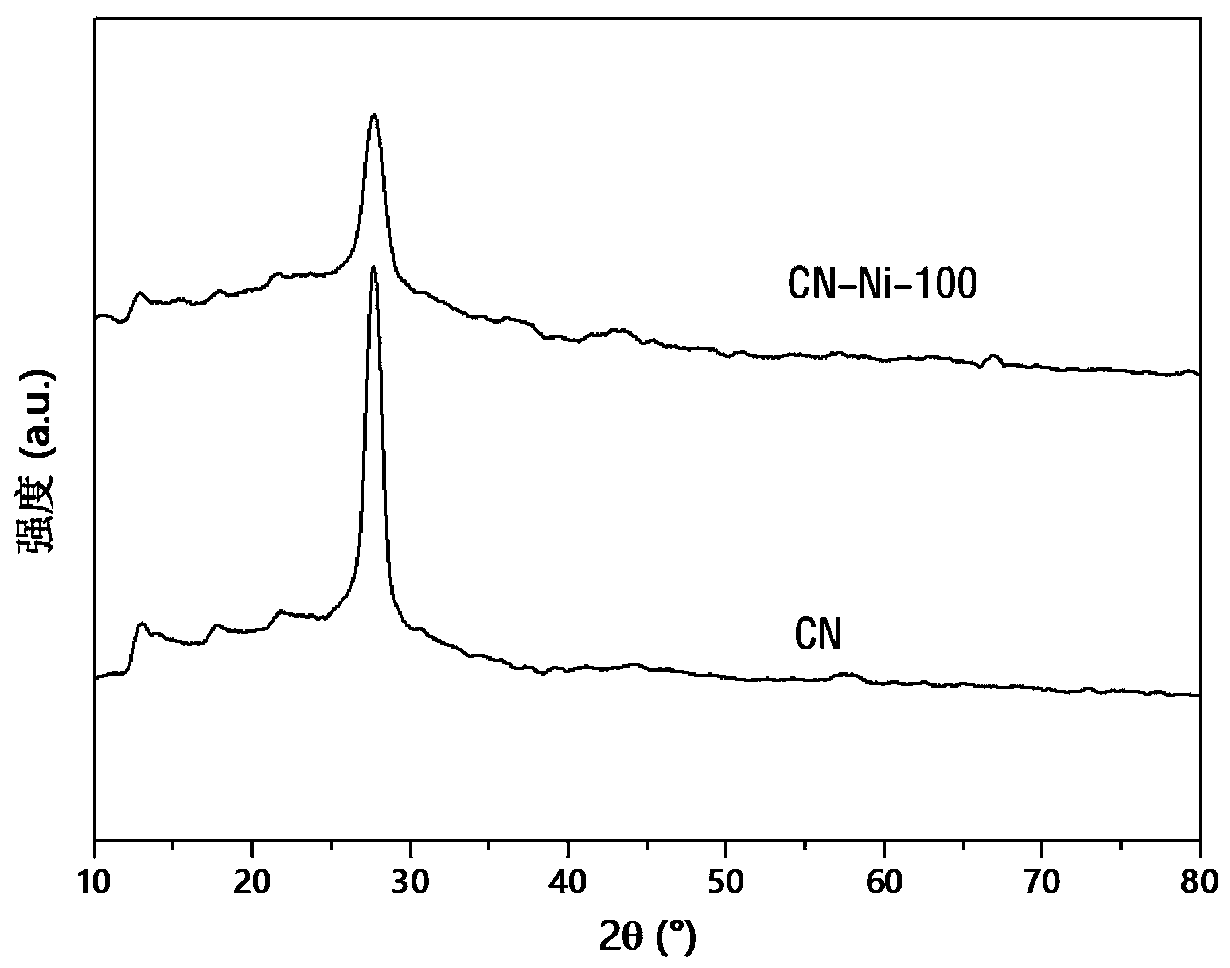
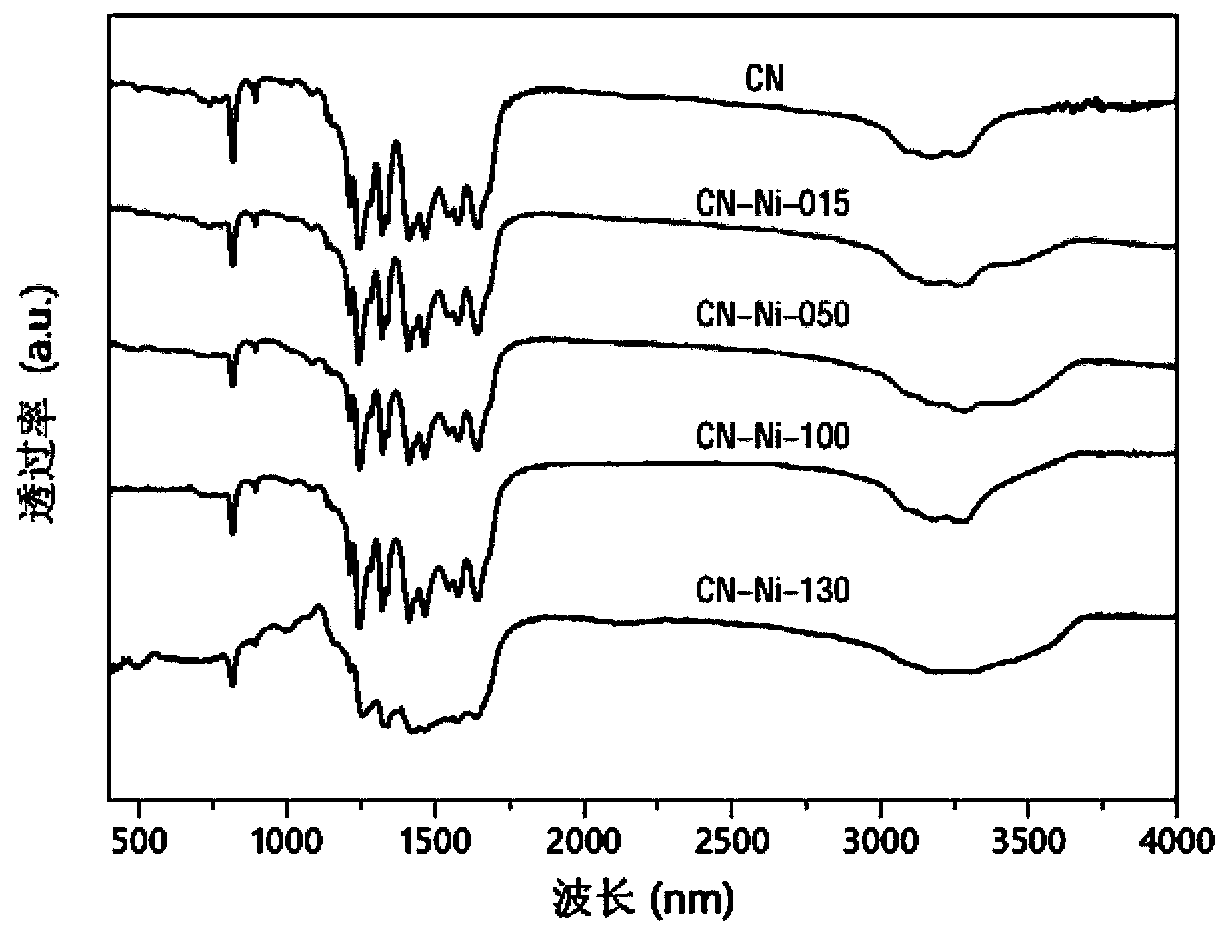
The invention relates to a Nickel single-active site graphite-phase carbon Nitride-based photocatalytic material as well as a preparation method and application thereof, in the photocatalytic material, Ni is dispersed in a g-C3N4 framework in a single-atom-level degree to form active sites, and the molar content of the Ni is 1-5%. the nickel single-active site graphite-phase carbon nitride-based photocatalytic material has a large specific surface area, is good in dispersion in an aqueous solution, and can effectively inhibit the recombination of photo-generated carriers, the reaction activitysites of the surface of the g-C3N4 are increased, the photocatalytic hydrogen production efficiency can be remarkably improved, and the photocatalytic reaction activity of the g-C3N4 can be improved.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
Doped quantum dot catalyst, preparation method thereof, hydrogen production system comprising doped quantum dot catalyst, and hydrogen production method
ActiveCN105478148AUniform sizeGood dispersionHydrogenPhysical/chemical process catalystsQuantum dotAlloy
The invention discloses a quantum dot catalyst doped with metal ions. The catalyst includes a light-harvesting unit and a catalyzing unit, wherein the light-harvesting unit includes one, two or several kinds of quantum dots, and the catalyzing unit comprises the metal ions doped in the quantum dots. The metal ions are dispersed on the quantum dots in the following one or several manners that: (1) the metal ions are adhered to the surface of the quantum dots; (2) the metal ions are uniformly dispersed in the quantum dots; (3) the metal ions are disposed in the quantum dots in a gradient alloy way; (4) the metal ions are merely disposed on cores of core-shell quantum dots; (5) the metal ions are merely disposed on shells of the core-shell quantum dots; (6) both the cores and shells of the core-shell quantum dots are doped with the metal ions. The invention further discloses a photocatalytic hydrogen production system of doped quantum dots. The system has high efficiency of a quantum-dot-catalyst photocatalytic hydrogen production system and simplicity of a single quantum-dot photocatalytic hydrogen production system. The system can conveniently combine with an oxygen production half-reaction to totally decompose water.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
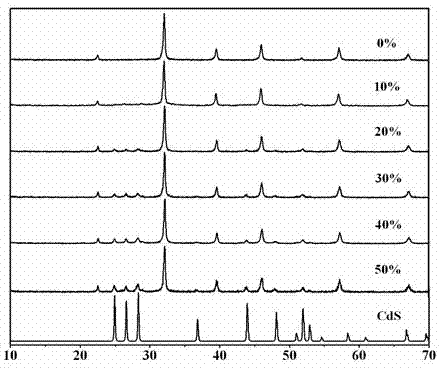
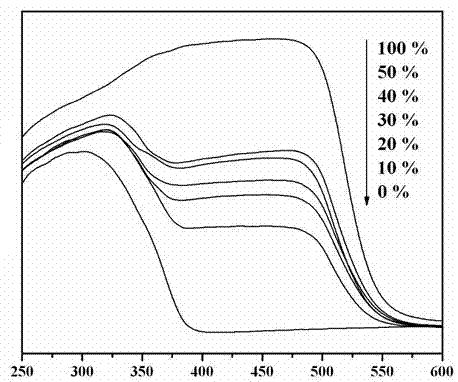
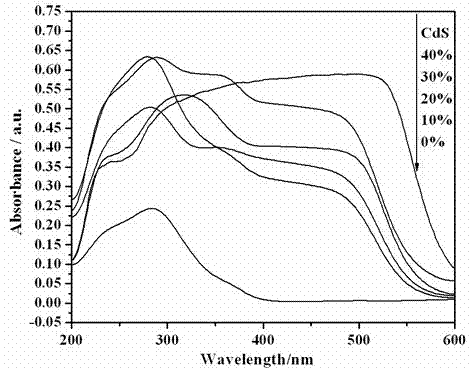
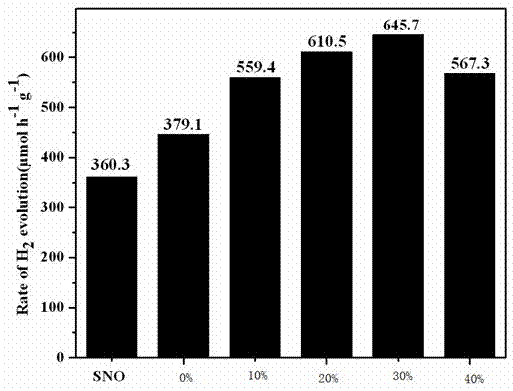
Photocatalytic water-splitting hydrogen production material CdS/Sr1.6Zn0.4Nb2O7 and preparation method thereof
ActiveCN103521244AEasy to operateHigh purityPhysical/chemical process catalystsHydrogen productionHeterojunctionPhotocatalytic water splitting
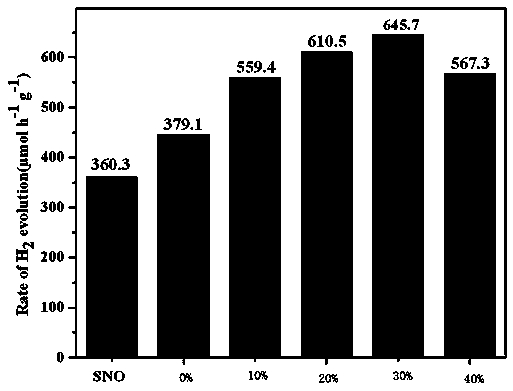
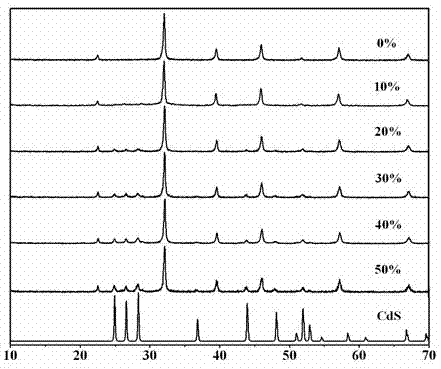
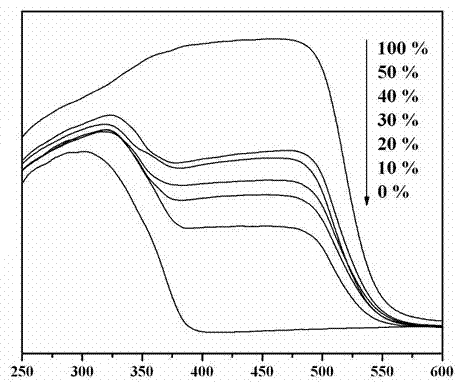
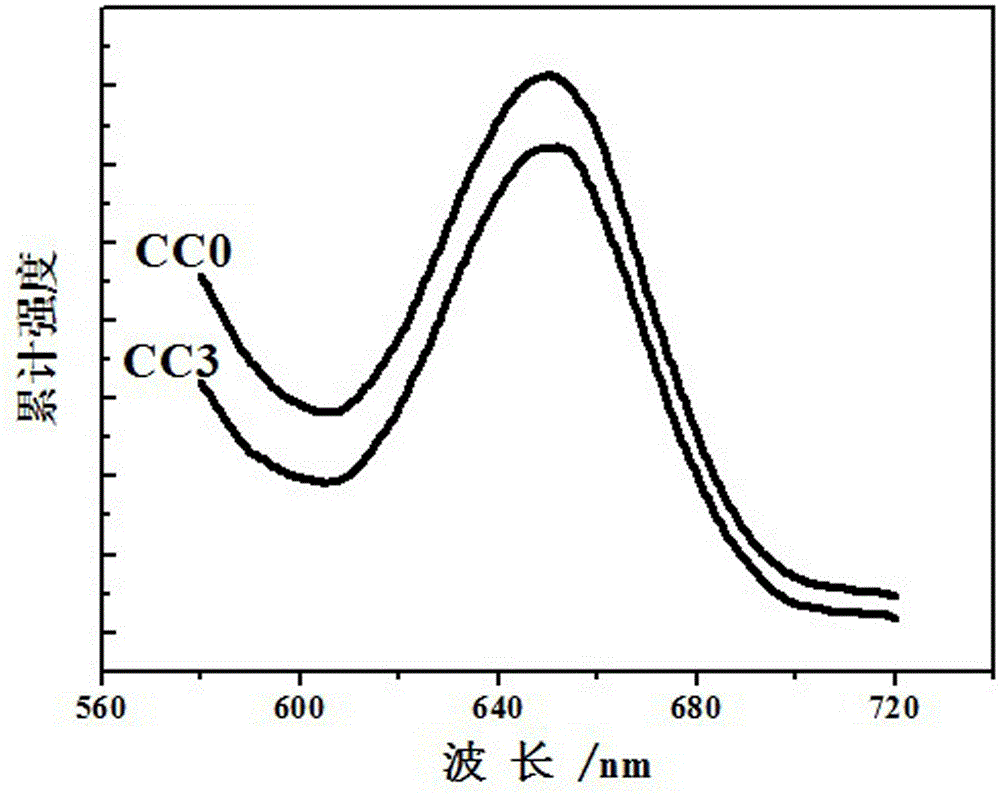
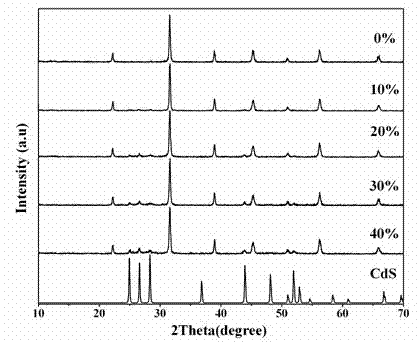
A photocatalytic decomposing water hydrogen production material CdS / Sr1.6Zn0.4Nb2O7 is a catalyst having a heterojunction structure formed by loading CdS on Sr1.6Zn0.4Nb2O7. The inventive catalyst is prepared by a hydrothermal method through sol gel. When a molar ratio of the CdS and the Sr1.6Zn0.4Nb2O7 is 3:7, the expression is 30%CdS / Sr1.6Zn0.4Nb2O7, and the catalytic effect of the material is best. Under the illumination of a 300 watt xenon lamp, Na2S / Na2SO3 is taken as a sacrifice reagent, the inventive material 30%CdS / Sr1.6Zn0.4Nb2O7 photocatalyzes and decomposes the water to produce the hydrogen without a co-catalyst of noble metal, and the efficiency is 645.7 mu mol.h<-1>.g<-1>. The photocatalytic decomposing water hydrogen production material of the invention has the advantages that: 1 the inventive catalyst is directly synthesized by the hydrothermal method through sol gel, the operation is simple, the production cost is low, the synthetic yield is higher, the purity is high and the repeatability is good, and the inventive catalyst is suitable for the demand of magnification production; 2, the inventive catalyst is good in the stability and is easy to reuse; and 3, the inventive catalyst has higher photocatalytic hydrogen production efficiency.
Owner:NANCHANG HANGKONG UNIVERSITY
Platinum/titanium dioxide nano flower composite material as well as preparation method and application thereof
ActiveCN108671907ALow hydrogen overpotentialReduce manufacturing costMetal/metal-oxides/metal-hydroxide catalystsPlatinumOxygen vacancy
The invention discloses a method for preparing a platinum / titanium dioxide nano flower composite material. The platinum / titanium dioxide nano flower composite material is prepared from titanium dioxide nanoflowers and platinum nanoparticles through compounding, and the titanium dioxide nanoflowers are adopted to provide a large specific area and are rich in oxygen vacancy. By virtue of the property that the oxygen vacancy has reducibility, the platinum nanoparticles are uniformly deposited to the surface of the titanium dioxide nanoflowers, and the platinum nanoparticles and the titanium dioxide nanoflowers are in tight interface contact. The platinum / titanium dioxide nano flower composite material prepared by using the method is an efficient and stable photoelectric converting material, aone-step simple reduction method is implemented, a platinum ion reduction process does not involve any reducing agents, and the preparation method is simple to operate, environmental-friendly, gentlein reaction condition, low in energy consumption and easy to popularize and use.
Owner:ZHEJIANG UNIV CITY COLLEGE
Method for preparing zinc oxide/zinc sulfide nano heterojunction photocatalyst
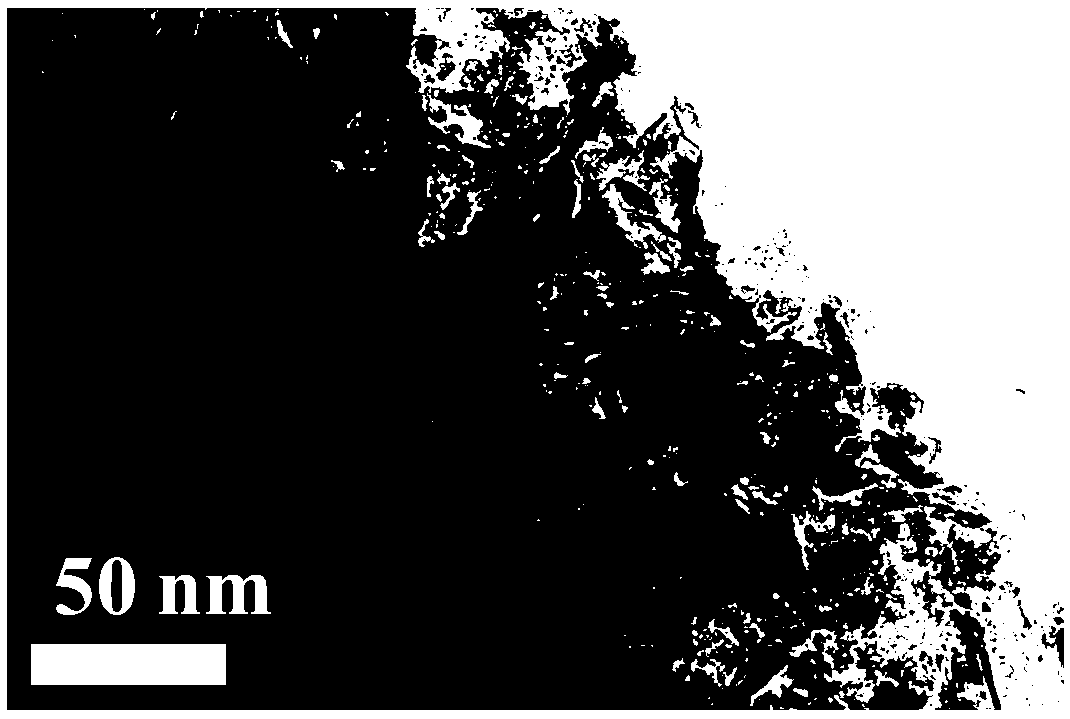
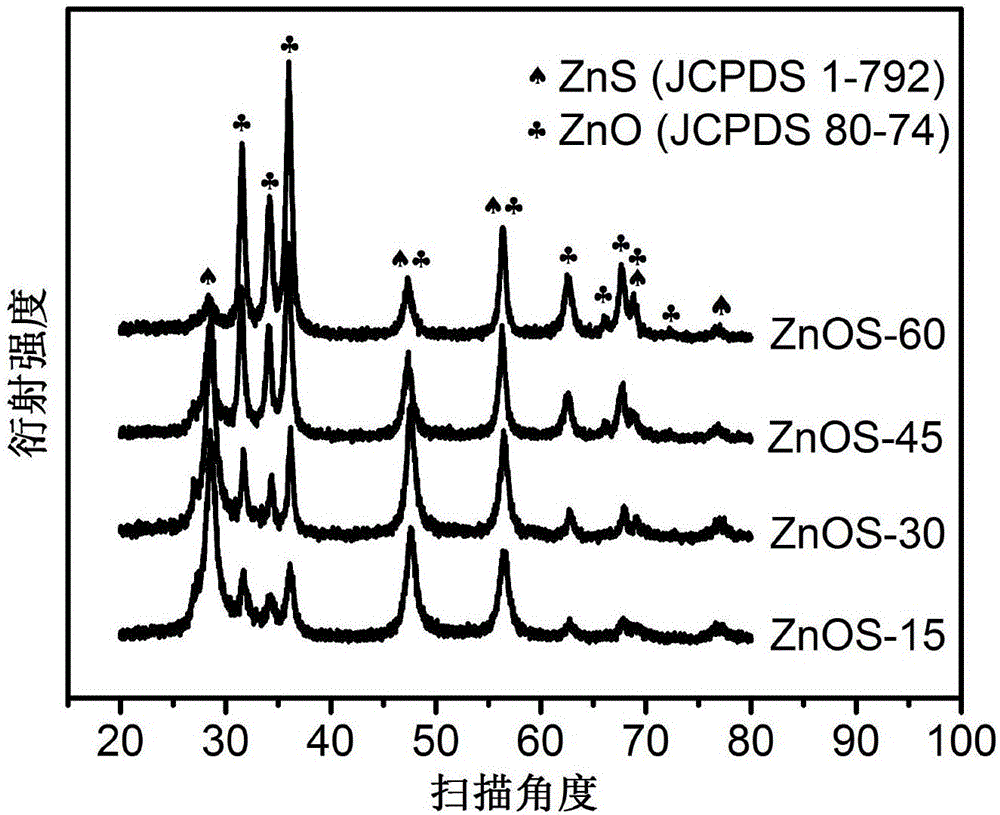
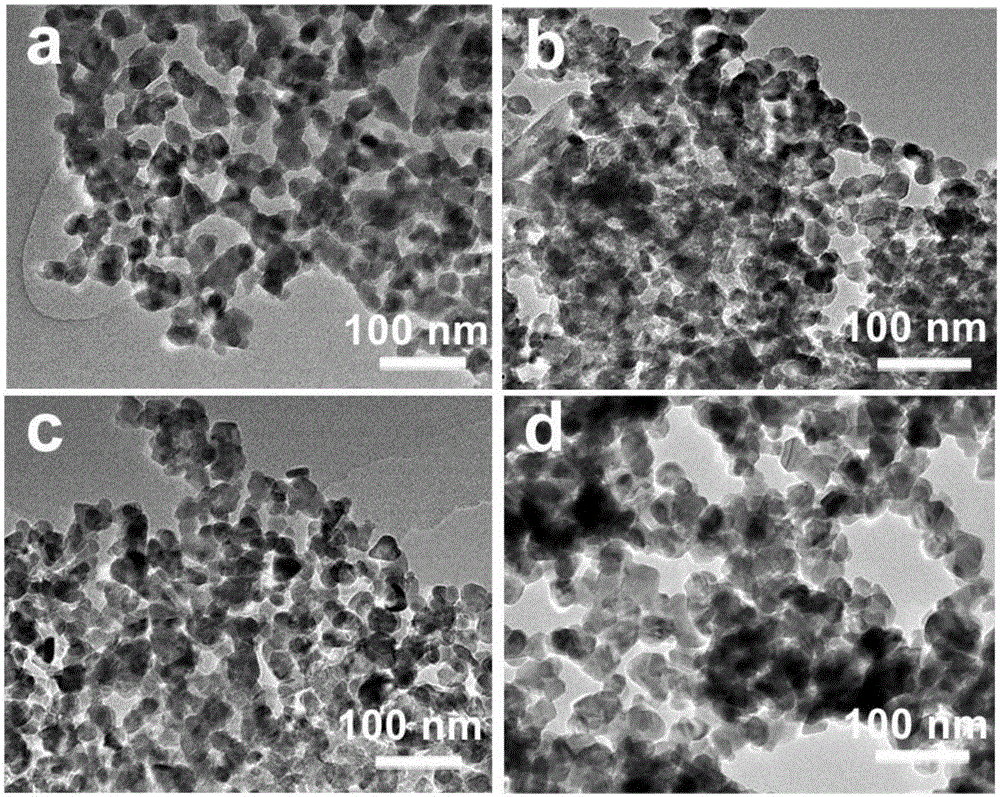
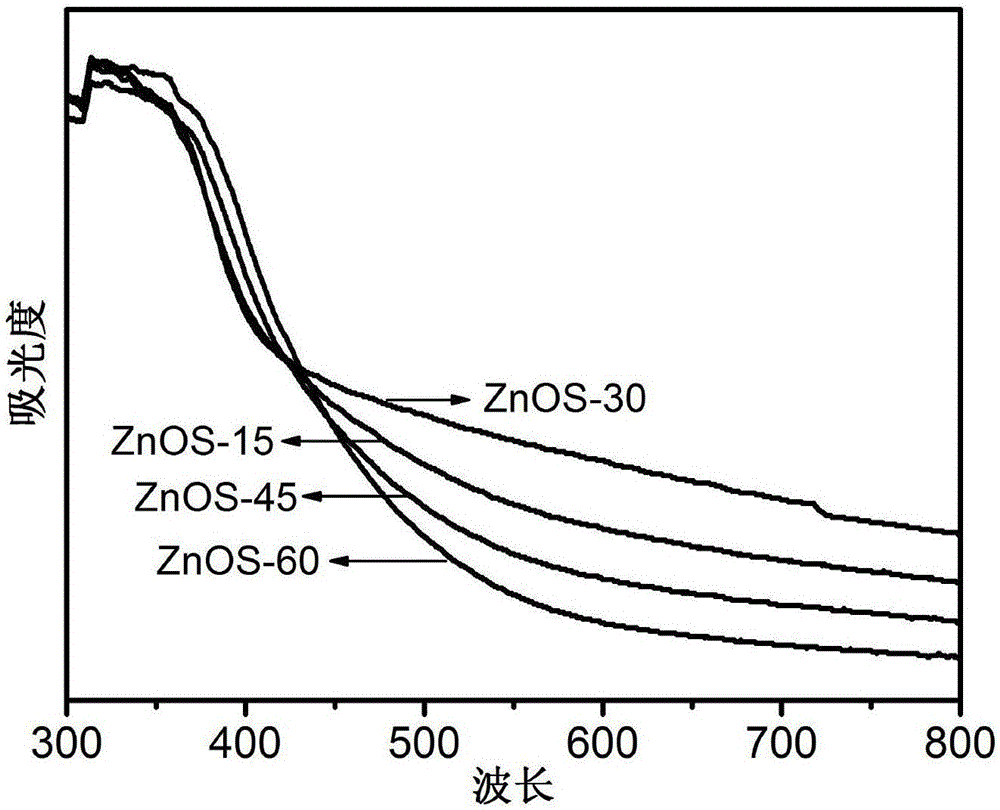
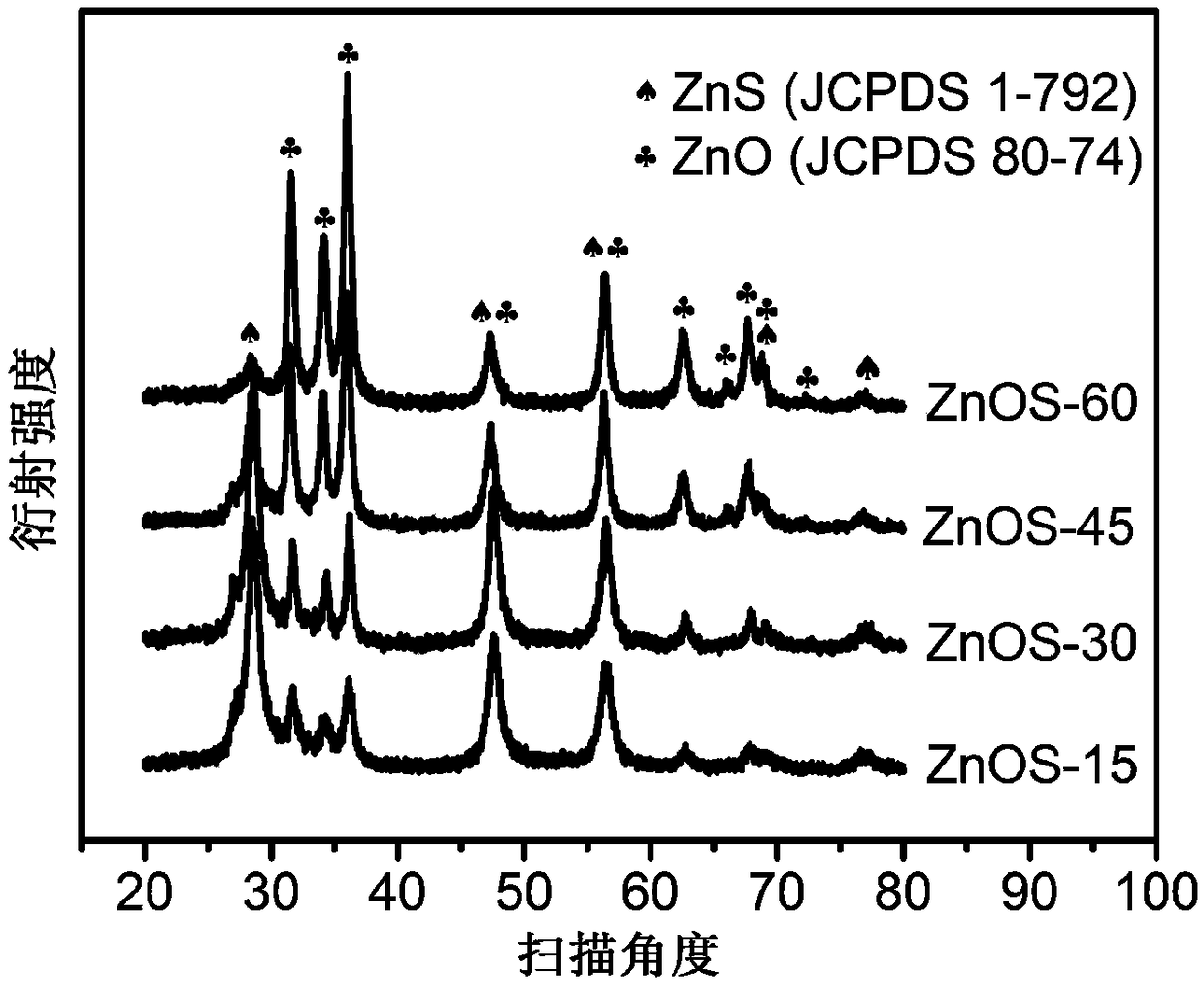
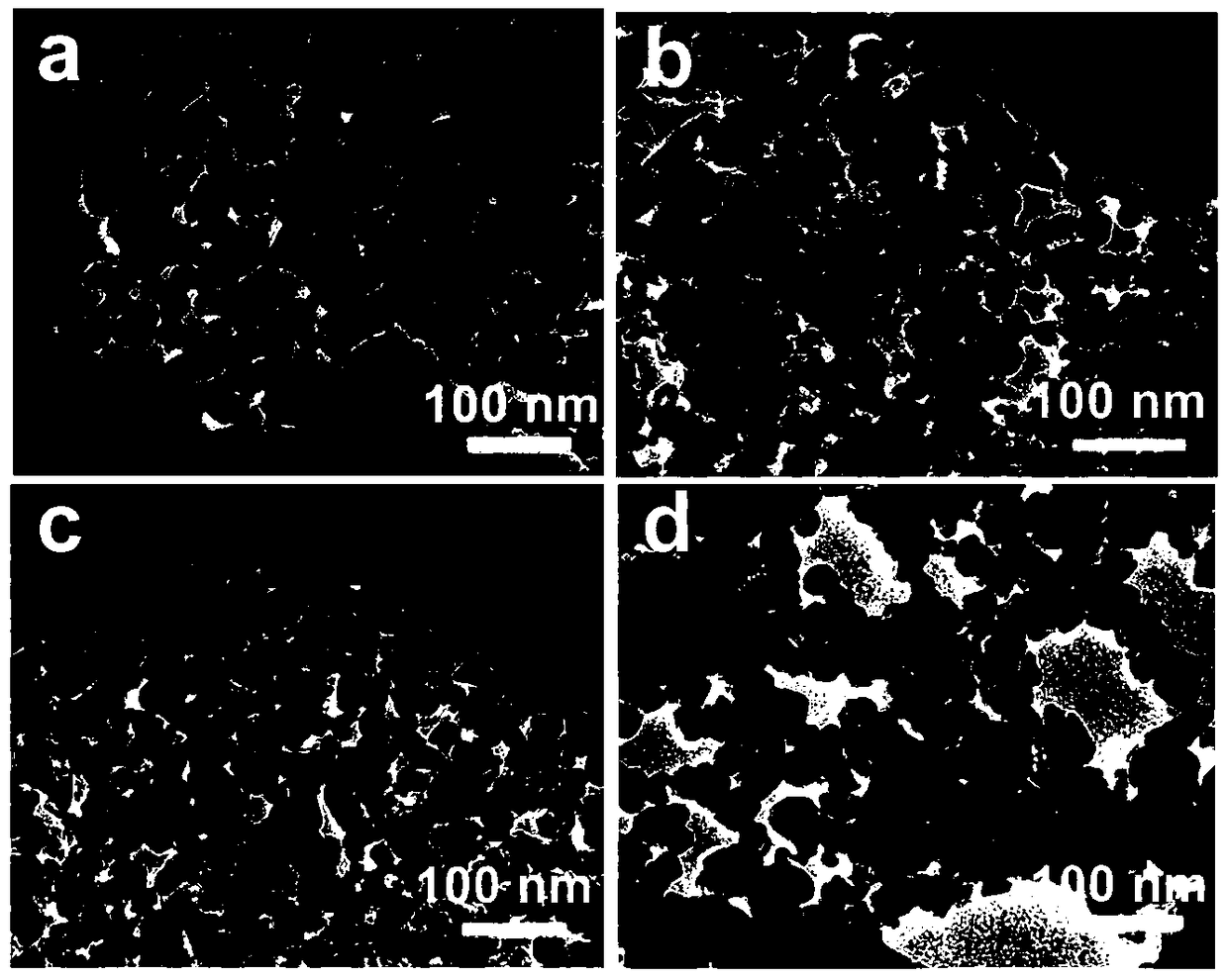
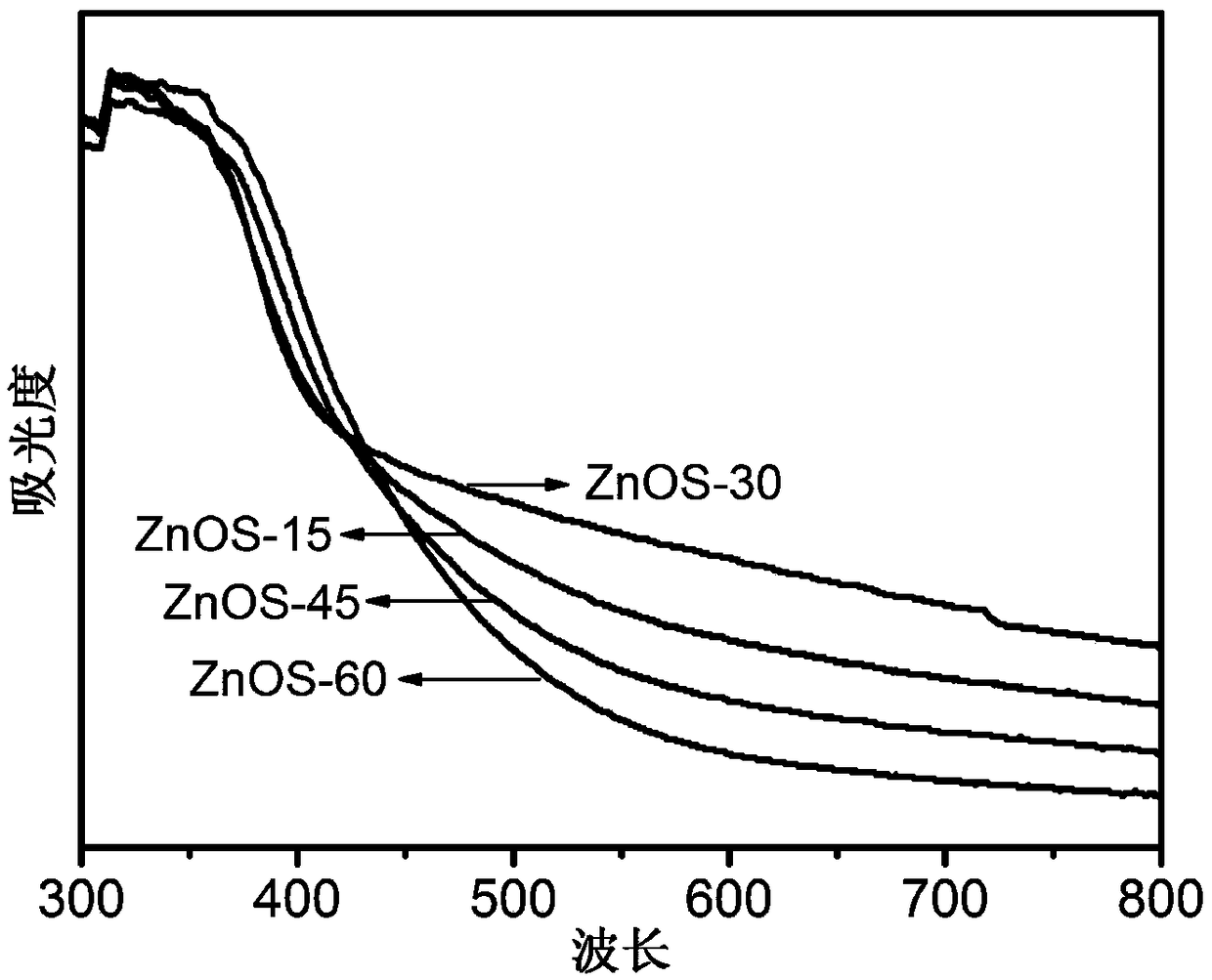
ActiveCN106622291ALarge-scale synthesisImprove photocatalytic hydrogen production efficiencyPhysical/chemical process catalystsHeterojunctionZinc sulfide
The invention provides a method for preparing a zinc oxide / zinc sulfide nano heterojunction photocatalyst. The catalyst is a heterojunction catalyst formed by loading ZnO to ZnS, and is formed through a two-step calcination method employing MOF-5 as a template, and an expression of the catalyst is ZnOS-x when ZnS-C is calcined in air for x minutes. The method has the advantages that (1) a heterojunction is firstly prepared by adopting a metal-organic frame complex as the template, and the catalyst prepared by the method compared with the prior art can reach a nanoscale and can be synthesized on a large scale; (2) the prepared catalyst is high in photocatalytic hydrogen production efficiency in visible light under the condition of not needing any co-catalyst; and (3) the prepared catalyst is good in stability and repeated utilization is facilitated.
Owner:NANKAI UNIV
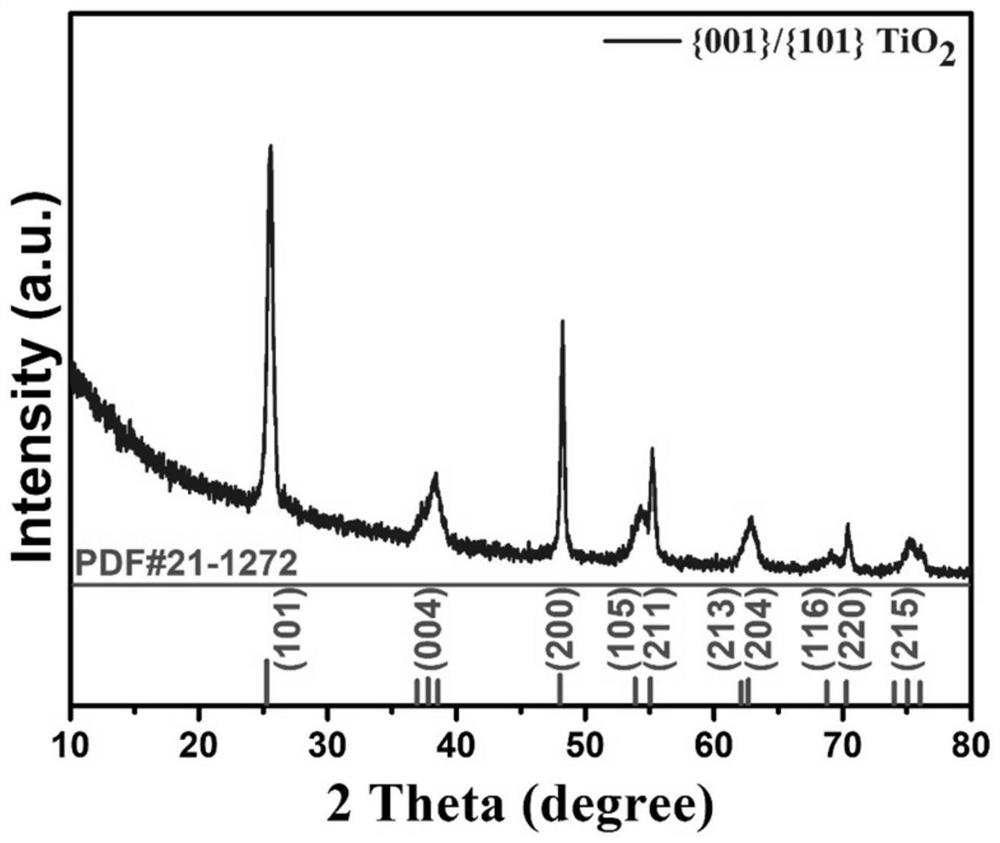
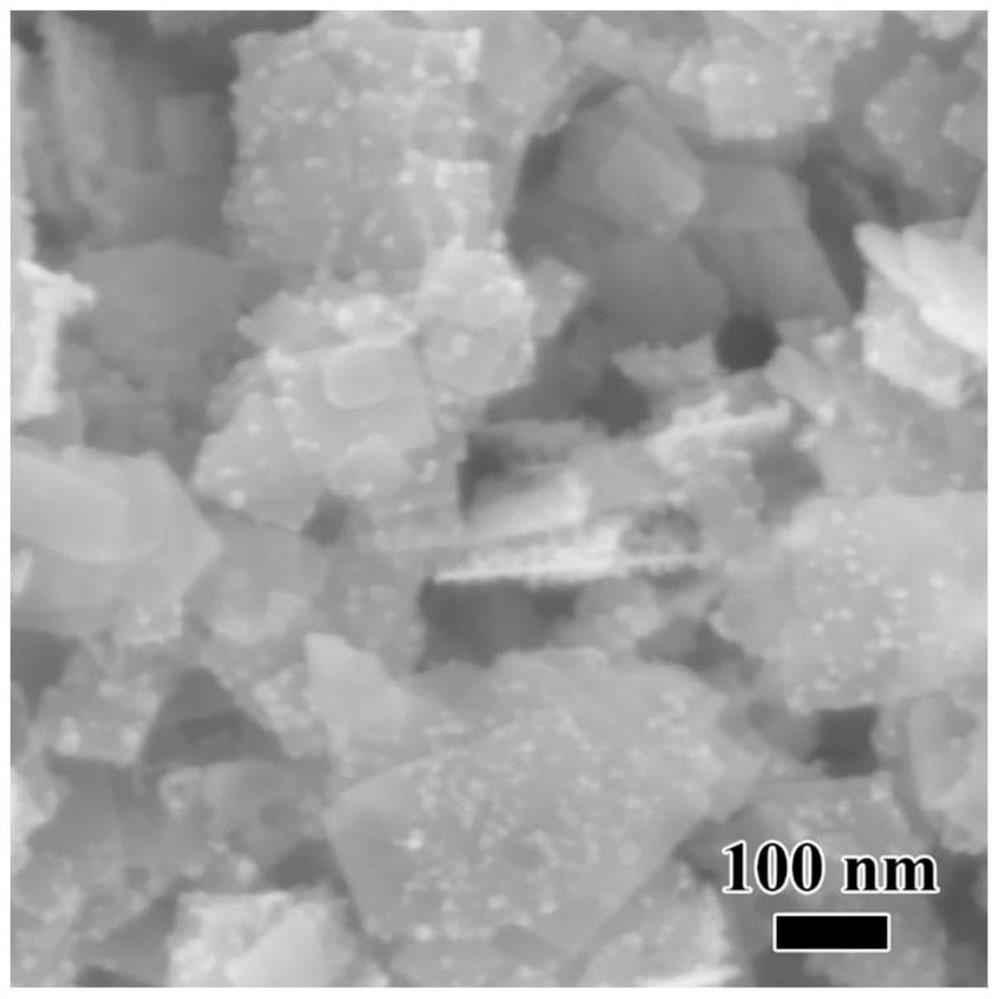
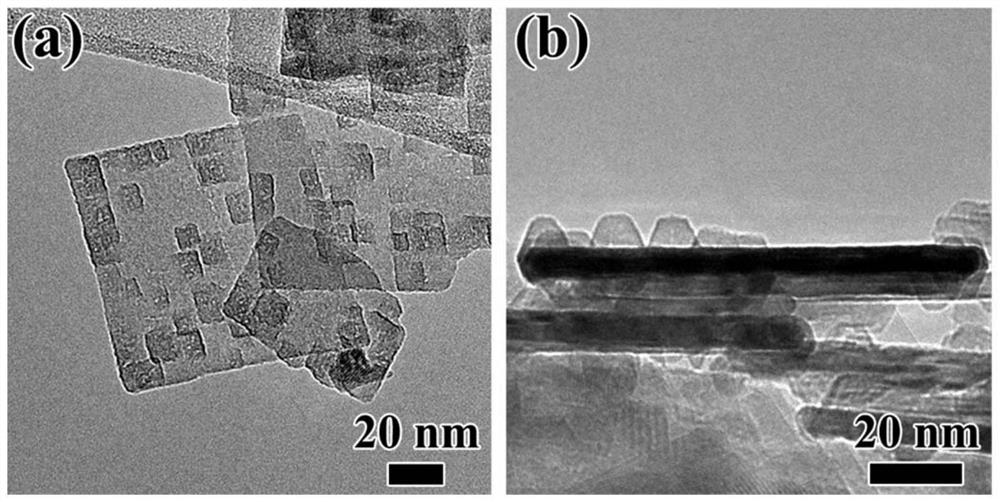
Titanium dioxide-platinum-tricobalt tetraoxide tri-element composite photocatalytic material and preparation method thereof
ActiveCN105664969AElectronic highImprove efficiencyHydrogen productionMetal/metal-oxides/metal-hydroxide catalystsElectron holePlatinum
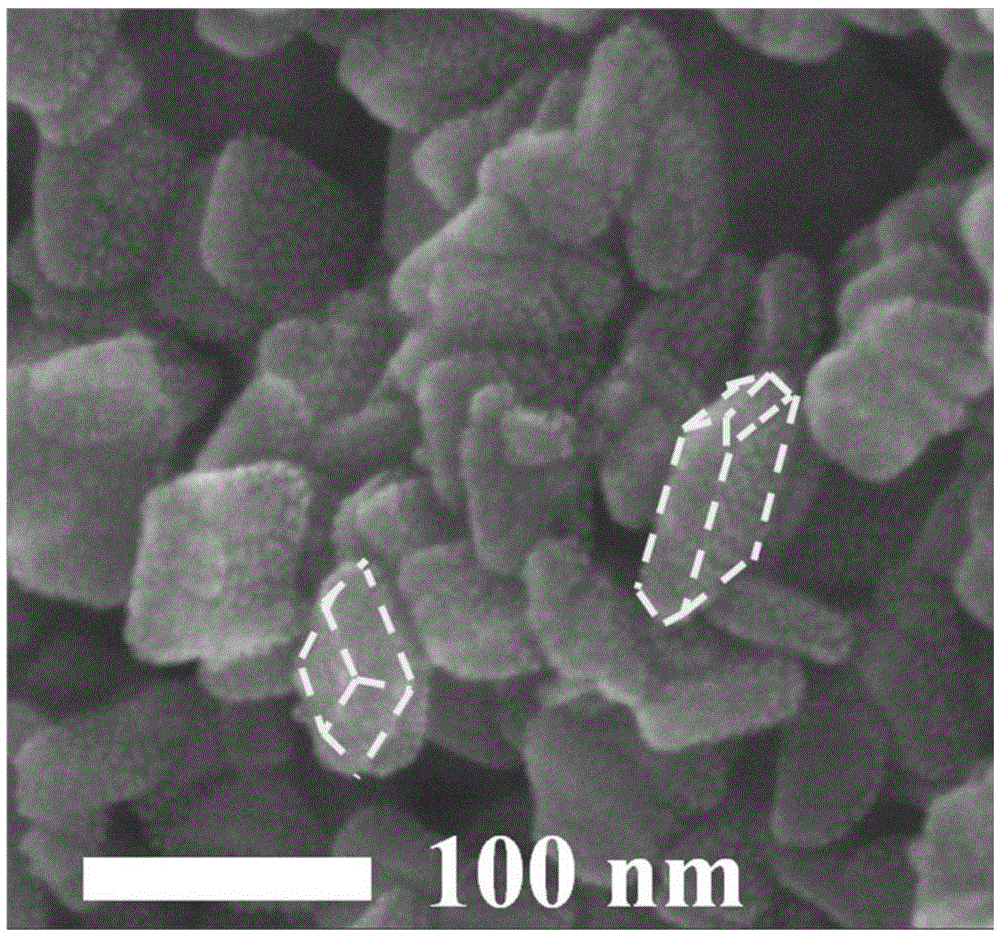
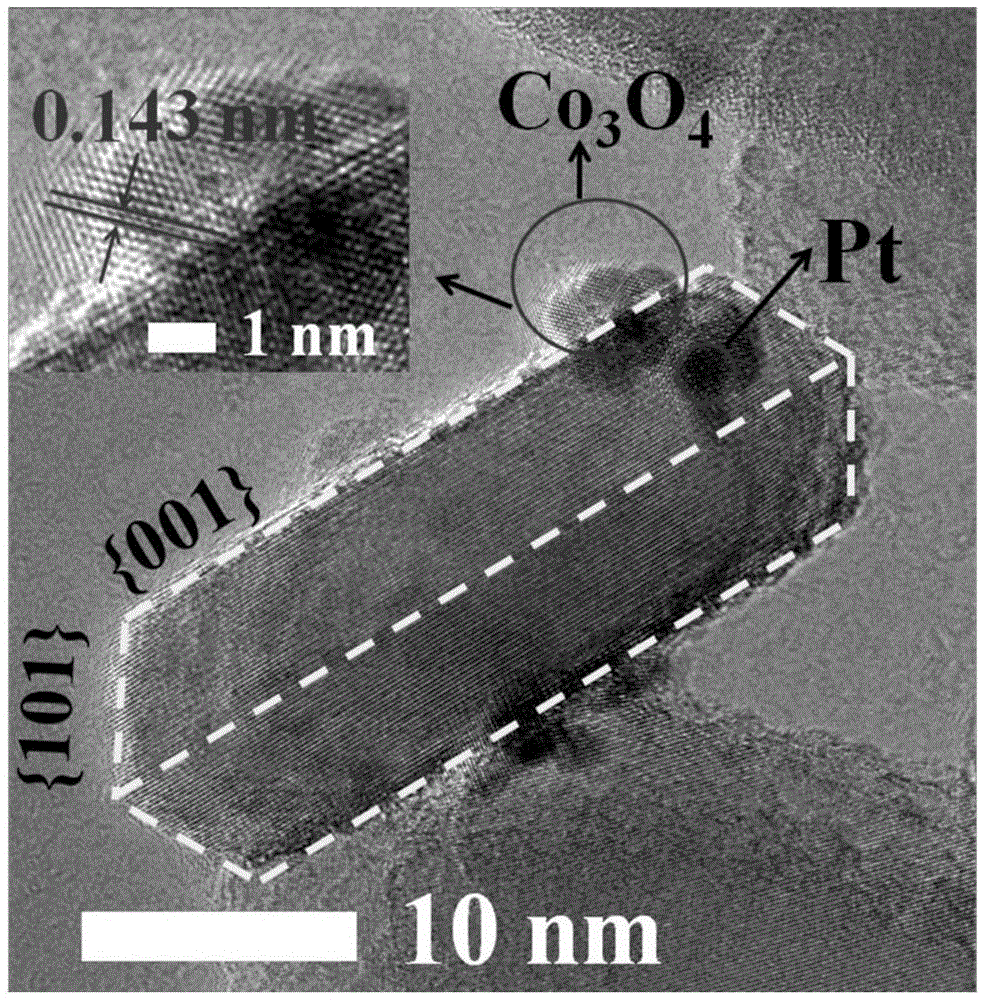
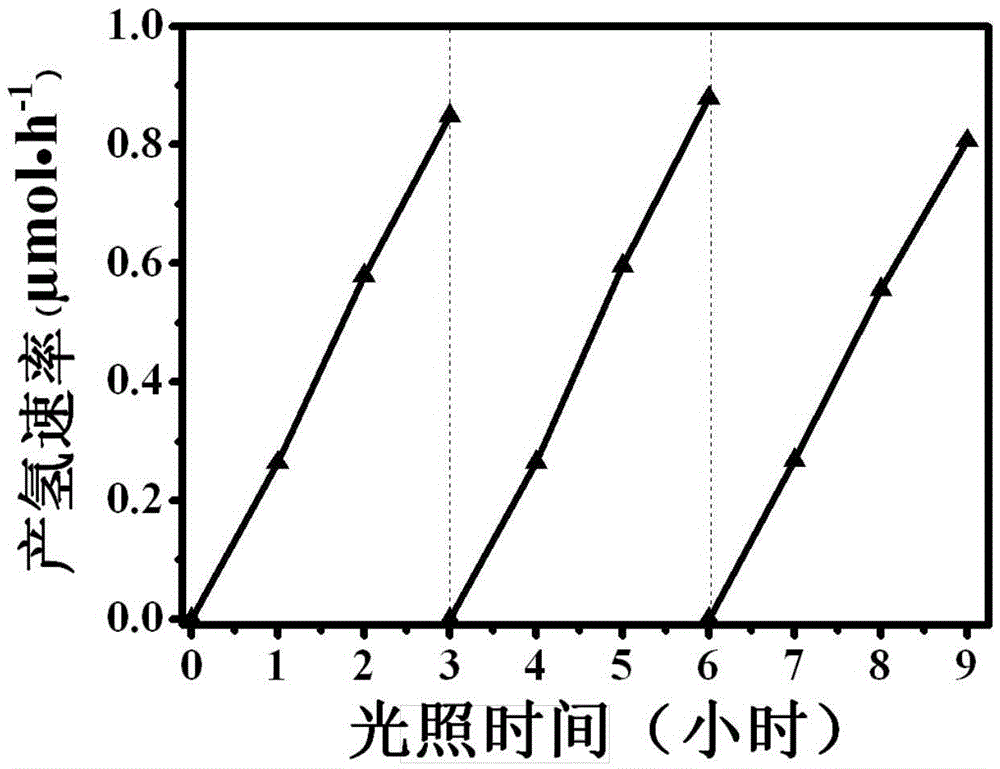
The invention relates to a titanium dioxide-platinum-tricobalt tetraoxide tri-element composite photocatalytic material and a preparation method thereof.Titanium dioxide nanosheets with exposed faces {001} and {101} are used as a carrier, platinum and tricobalt tetraoxide nanoparticles are used as a co-catalyst, the platinum particles are loaded on the faces {101} of the titanium dioxide nanosheets, and the tricobalt tetraoxide nanoparticles are loaded on the faces {001} of the titanium dioxide nanosheets.Photocatalytic decomposing water of the material is high in hydrogen generating efficiency, and the co-catalyst and titanium dioxide are combined closely, so that the material is high in stability after long-time use and the problem that photocatalytic efficiency is lowered caused by the fact that photocatalyst electron holes are highly prone to compositing in the prior art is solved effectively.
Owner:WUHAN UNIV OF TECH
Adsorption photocatalytic hydrogel material and application thereof in synergistic photocatalytic sewage hydrogen generation capable of reversing heavy metal pollution
InactiveCN107349965AStrong water absorptionReduce concentrationOther chemical processesOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenPhysical chemistry
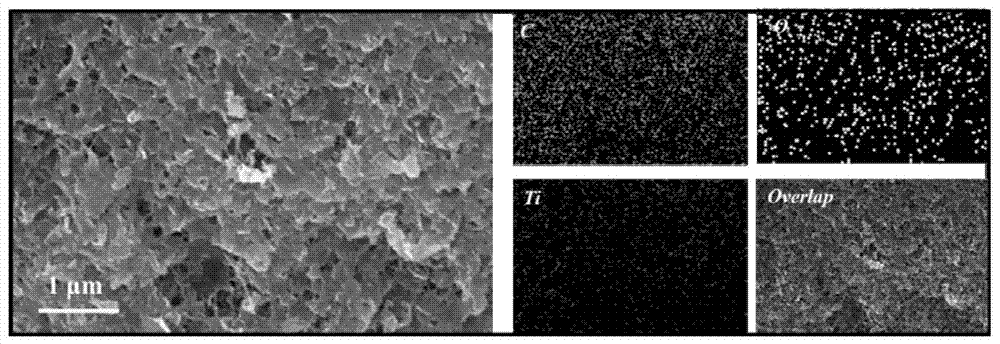
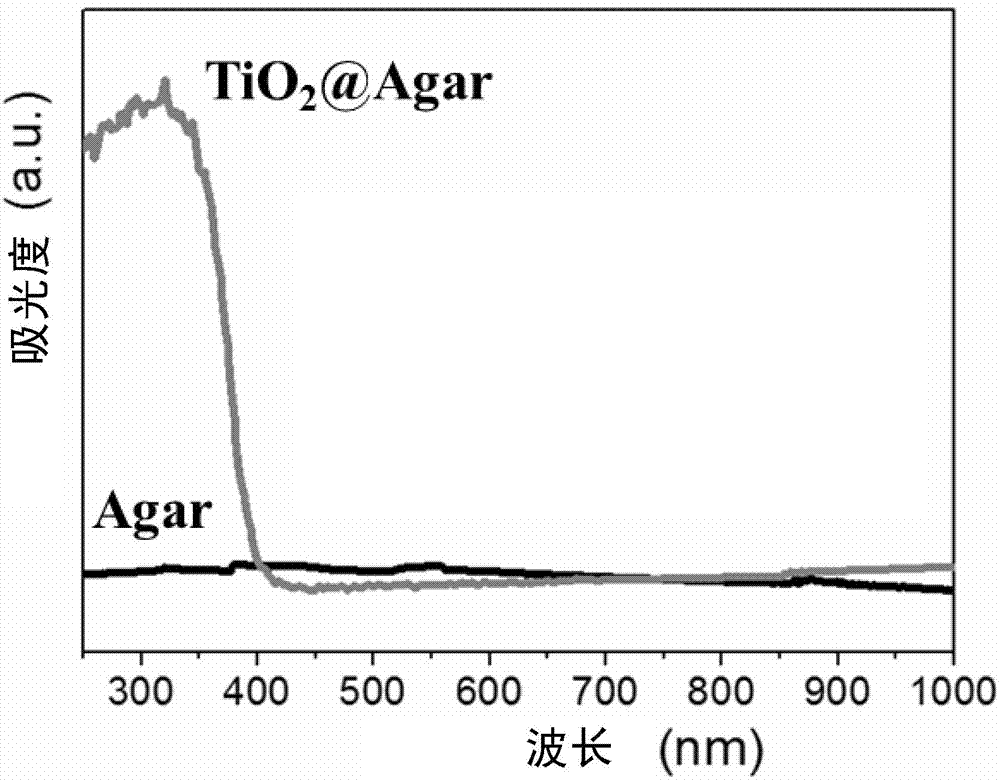
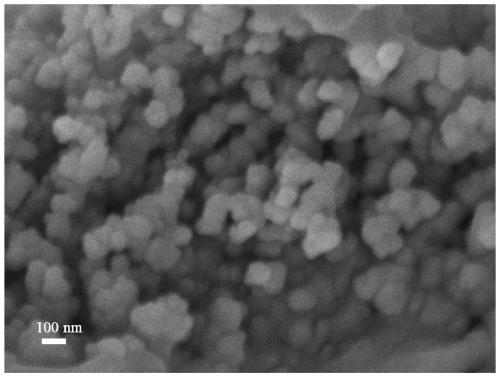
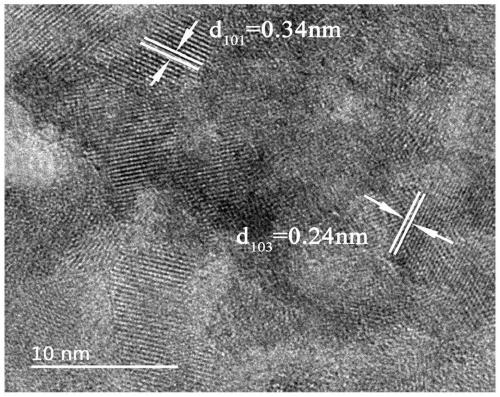
The invention relates to an adsorption photocatalytic hydrogel material and an application thereof in synergistic photocatalytic sewage hydrogen generation capable of reversing heavy metal pollution. The adsorption photocatalytic hydrogel material comprises a photocatalytic nano material and a porous transparent matrix for loading the photocatalytic nano material. The porous transparent matrix is prepared from a polysaccharide polymer material, which carry out crosslinking easily to form hydrogel. The polysaccharide hydrogel is rich in functional groups such as -OH, -NH3, and the like, can absorb heavy metal ions, and reduces the concentration of heavy metal ions in sewage.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
Preparation method and application of modified material based on titanium dioxide
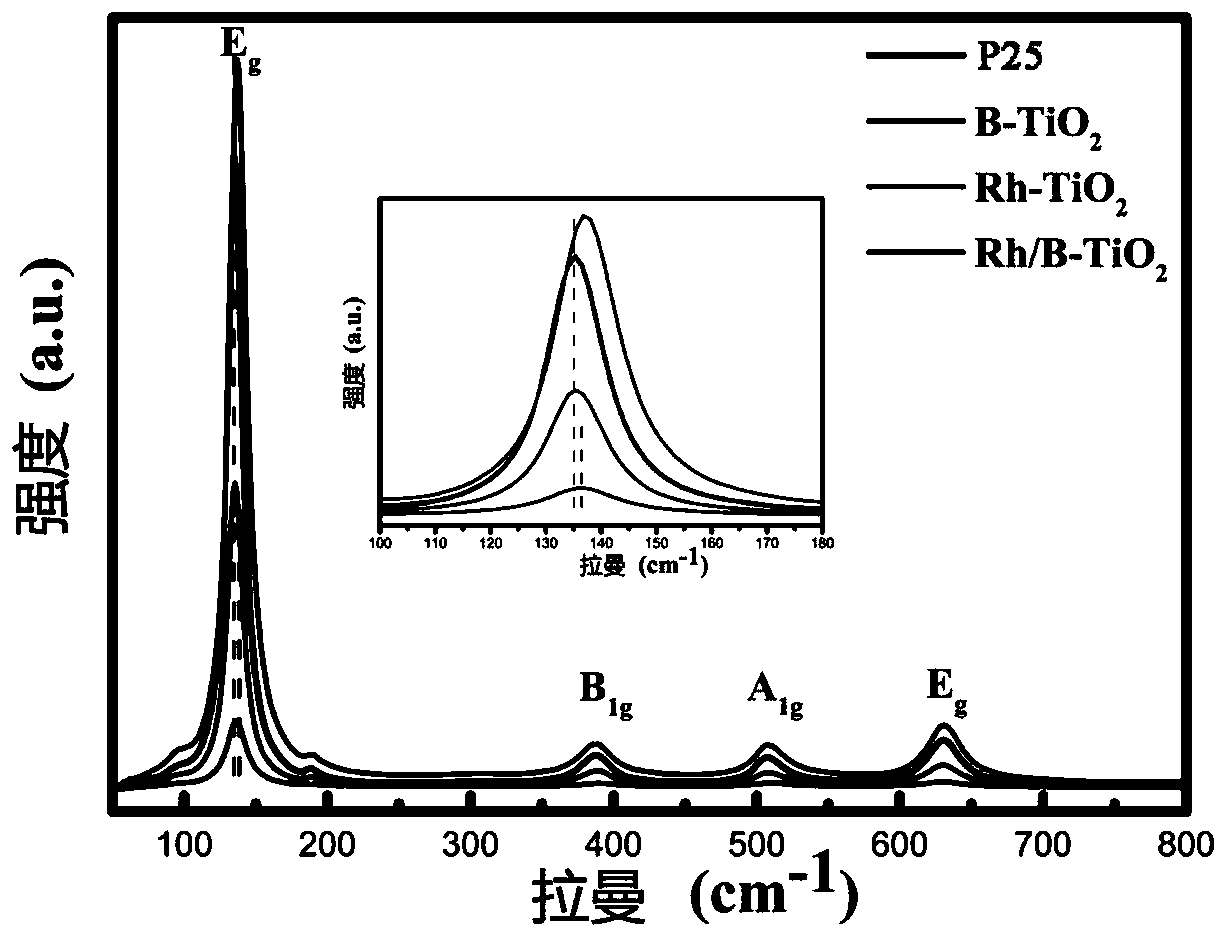
PendingCN111408368ABroaden the range of light absorptionImprove photocatalytic hydrogen production efficiencyHydrogen productionHydrogen/synthetic gas productionTitanium oxideMaterials science
The invention belongs to the technical field of materials, and provides a preparation method and application of a modified material based on titanium dioxide, which comprises the following steps: dissolving a certain amount of boric acid and rhodium chloride trihydrate in water containing nitric acid and alcohol to obtain a solution A; dropwise adding isopropyl titanate into the ethanol solution at the temperature of 0-4 DEG C to obtain a solution B; slowly dropwise adding the solution A into the solution B to obtain titanium dioxide sol; aging the titanium dioxide sol at room temperature, andperforming drying to obtain xerogel; and finally, grinding the dry gel into powder, and calcining the powder in air for 4 hours to obtain the boron-rhodium co-doped titanium dioxide, namely the titanium dioxide-based modified material. The material is used for photocatalytic hydrogen production, and the catalytic hydrogen production efficiency is effectively improved. The boron-rhodium co-doped titanium dioxide is prepared by adopting a one-pot sol-gel method, the absorption bandwidth of the titanium dioxide can be regulated and controlled by adjusting the rhodium doping proportion, and the light absorption and carrier separation efficiency is improved.
Owner:SHANGHAI UNIVERSITY OF ELECTRIC POWER
Composite photocatalyst and preparation method thereof
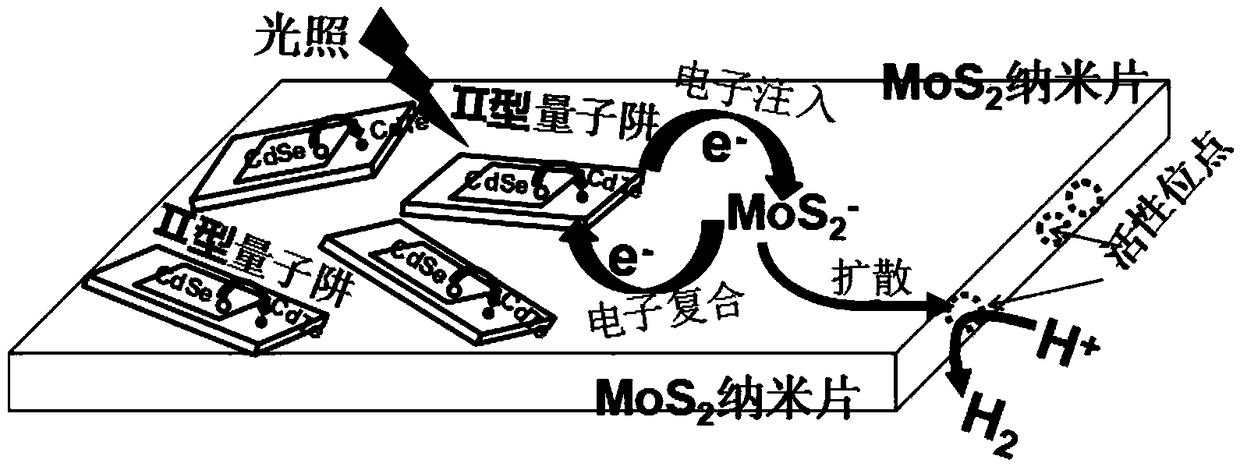
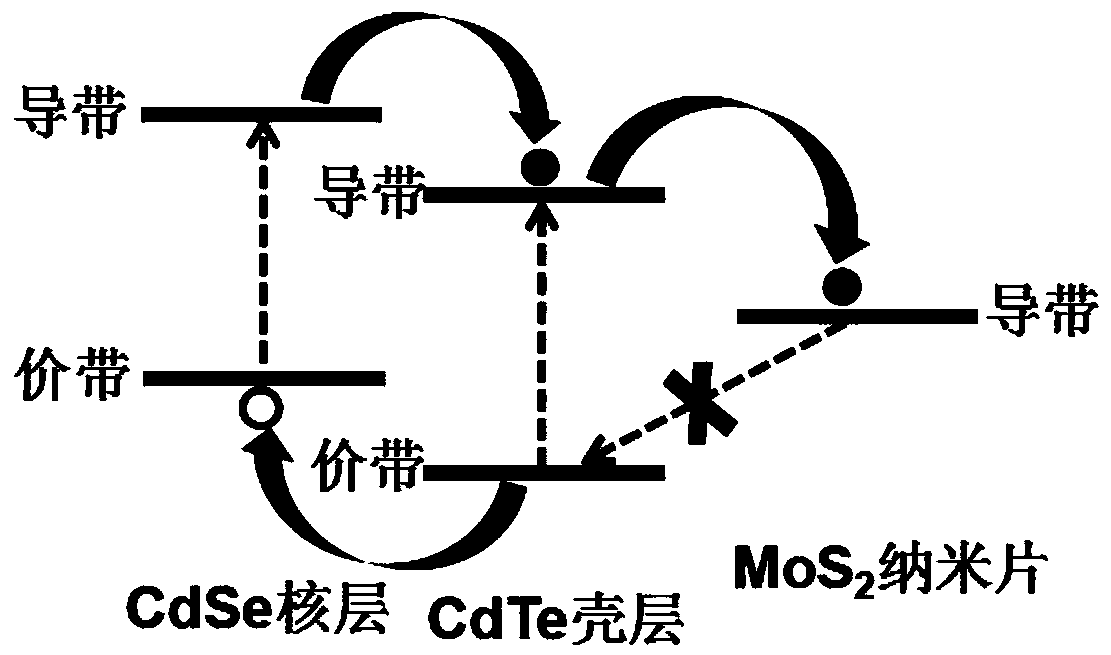
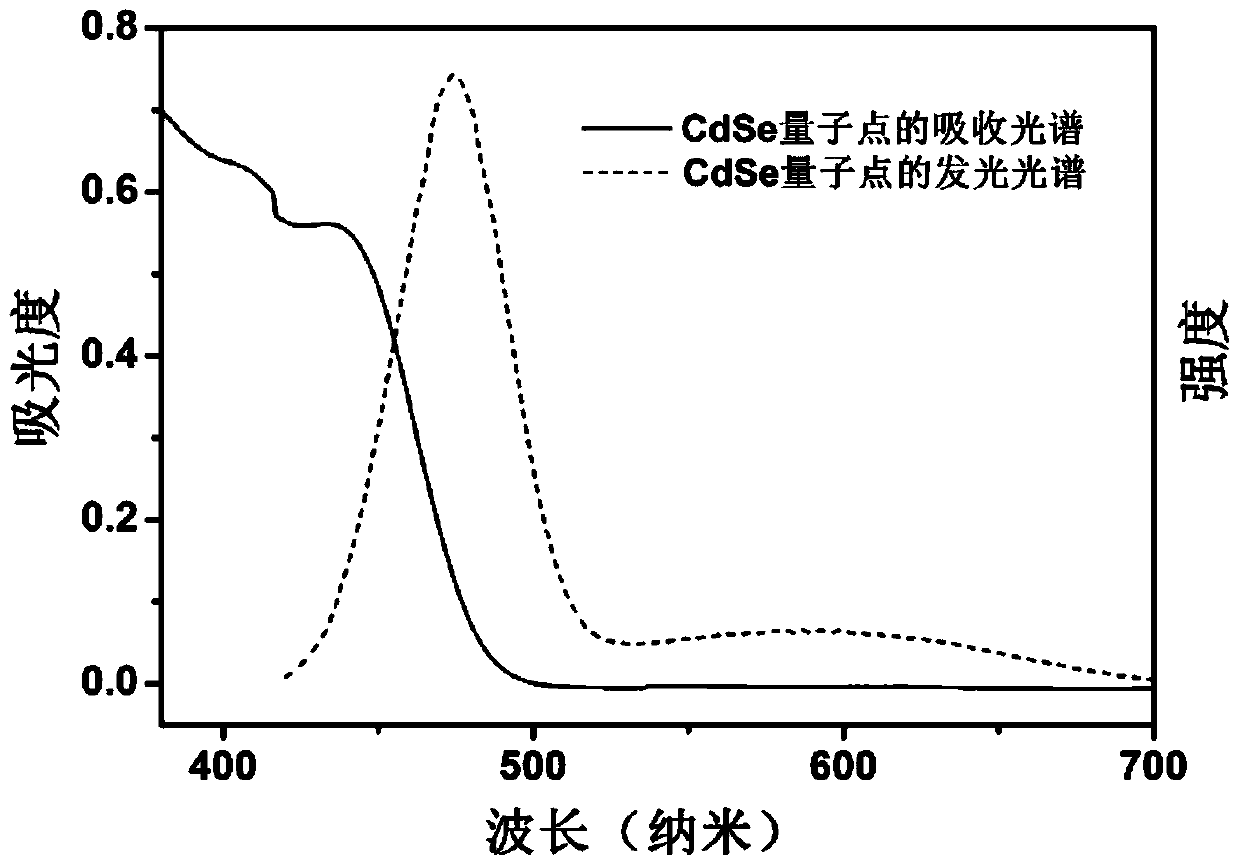
PendingCN108855149AImprove light absorption efficiencyImprove photocatalytic hydrogen production efficiencyPhysical/chemical process catalystsHydrogen productionQuantum yieldQuantum well
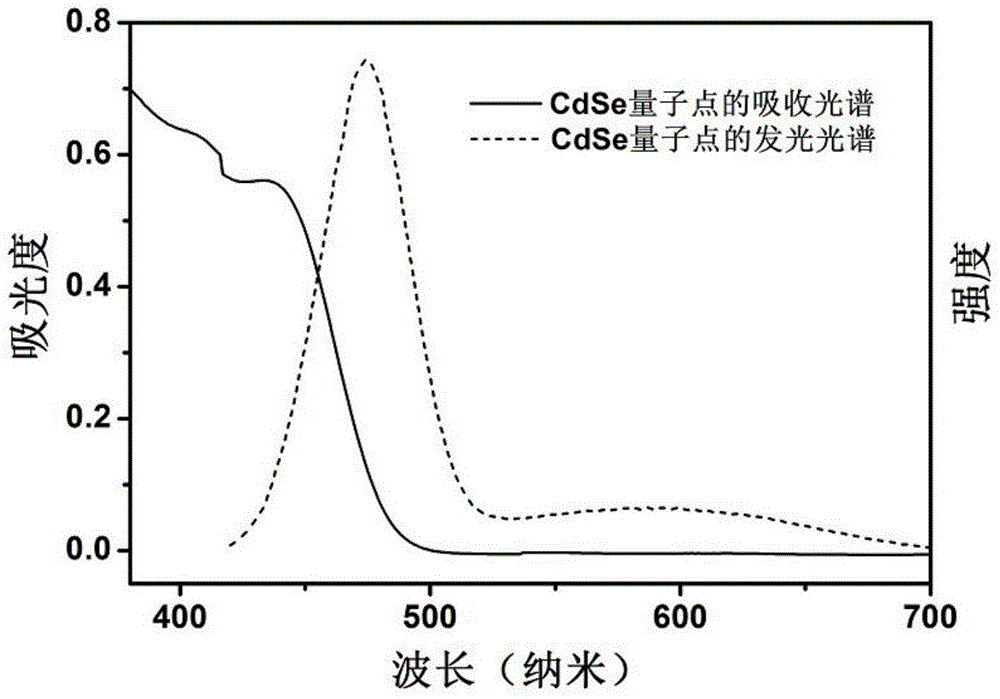
The invention discloses a composite photocatalyst and a preparation method thereof, and belongs to the field of photocatalysis. The composite photocatalyst comprises a MoS2 nanosheet and an II type CdSe / CdTe quantum well adsorbed to the MoS2 nanosheet, wherein the II type CdSe / CdTe quantum well comprises a CdSe quantum chip nuclear layer and a CdTe quantum chip shell layer. The composite photocatalyst has the advantages of high light absorption efficiency, high separation efficiency of interfacial charge, weak recombination action of the interfacial charge, high quantum yield of photocatalytichydrogen production and the like. The preparation method of the composite photocatalyst comprises the following steps: dropwise adding first reaction liquid containing the MoS2 nanosheet into secondreaction liquid containing the II type CdSe / CdTe quantum well and carrying out ultrasonic dispersion to obtain the composite photocatalyst. The preparation method has the advantages of good controllability, low cost and wide application range; the prepared photocatalyst has high light absorption efficiency and high hydrogen production efficiency.
Owner:HUBEI UNIV OF ARTS & SCI
Graphite phase carbon nitride foam photocatalytic material and preparation method thereof
ActiveCN109261191AEasy to makeWide variety of sourcesPhysical/chemical process catalystsHydrogen productionFreeze-dryingSlurry
The invention relates to a graphite phase carbon nitride foam photocatalytic material and a preparation method thereof. The preparation method includes adding 5-14 parts by mass of graphite phase carbon nitride to 100 parts by mass of deionized water, mixing, further adding 1.7-2.0 parts by mass of sodium dodecyl sulfate, 1.7-2.0 parts by mass of dodecyl alcohol and 1.7-2.0 parts by mass of resingum and stirring at 40-60 DEG C and 100-200 r / min for 10-20 minutes to obtain a mixed solution; stirring the mixed solution at 1500-2000 r / min for 15-20 min, then adding 5-14 parts by mass of binder and continuing stirring for 5-10 min to obtain graphite phase carbon nitride foam slurry; molding the graphite phase carbon nitride foam slurry by casting, freeze drying for 6-12 hours, and drying at 80-100 DEG C for 18-24 hours to obtain the graphite phase carbon nitride foam photocatalytic material. The preparation method has simple process and low cost. The prepared graphite phase carbon nitridefoam photocatalytic material has high photocatalytic hydrogen production efficiency.
Owner:WUHAN UNIV OF SCI & TECH
Preparation method and application of silicon carbide-based photocatalyst
InactiveCN109453798AIncrease the active siteIncrease surface areaPhysical/chemical process catalystsHydrogen productionPtru catalystVisible light photocatalytic
The invention discloses a silicon carbide-based double-additive hydrogen producing photocatalyst and a preparation method thereof. The preparation method comprises the following steps of a, performingpretreatment on silicon carbide; b, by taking molybdenum disulfide as a raw material, preparing 8 wt% molybdenum disulfide suspension liquid of a whole hydrothermal reaction system; c, putting 100 mLof 8 wt% molybdenum disulfide suspension liquid in a beaker, sequentially adding silicon carbide (SiC), graphene oxide (GO) and two drops of 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid, and performing ultrasonic stirring to enable the components to be uniformly mixed; and d, transferring the mixture into a high-temperature reaction kettle, performing reaction for 20 h at the temperature of 220 DEG C, then performing centrifugal washing until a pH value is 7, putting the product in a vacuum drying tank, performing vacuum drying for later use, and performing hydro-thermal synthesis to obtain the silicon carbide-based double-additive hydrogen producing photocatalyst. According to the preparation method provided by the invention, for the silicon carbide-based double-additivehydrogen producing photocatalyst prepared by utilizing the synergistic effect of GO-molybdenum disulfide (MoS2) double-additive catalyst, the performance of photocatalytically decomposing water with visible light to produce hydrogen is improved, and a certain foundation can be laid for subsequent efficient application of a visible light catalyst.
Owner:SHANDONG UNIV OF SCI & TECH
Graphene quantum dot modified manganese oxide/titanium oxide nanotube array material and preparation method and application thereof
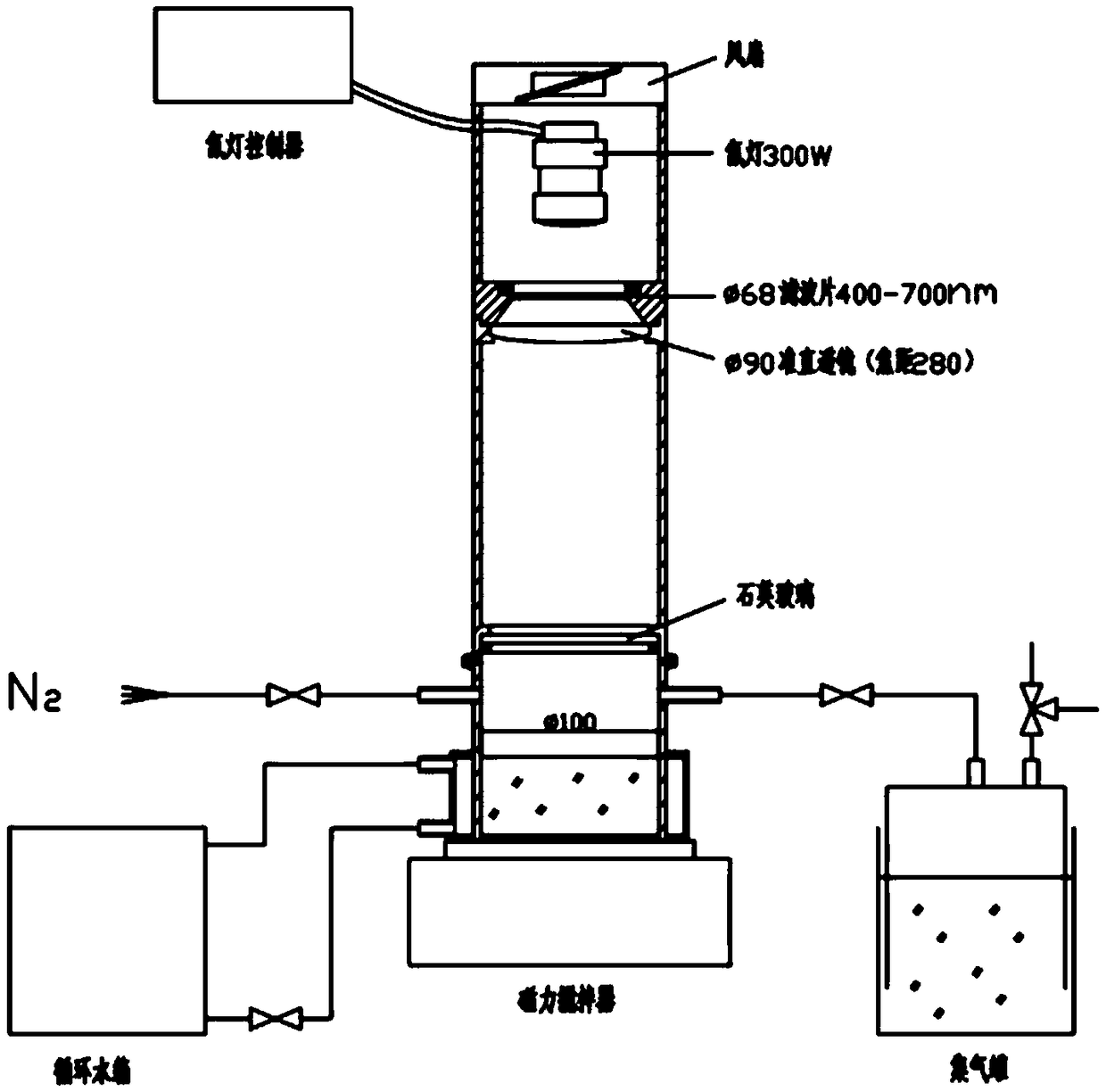
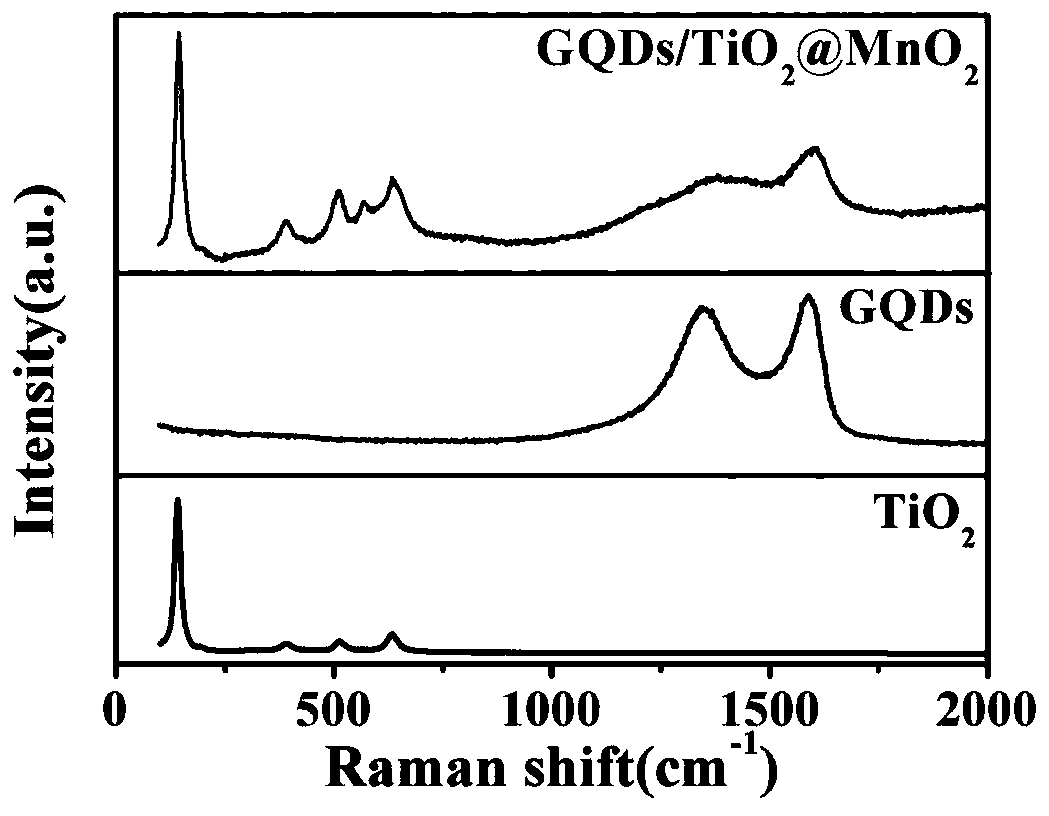
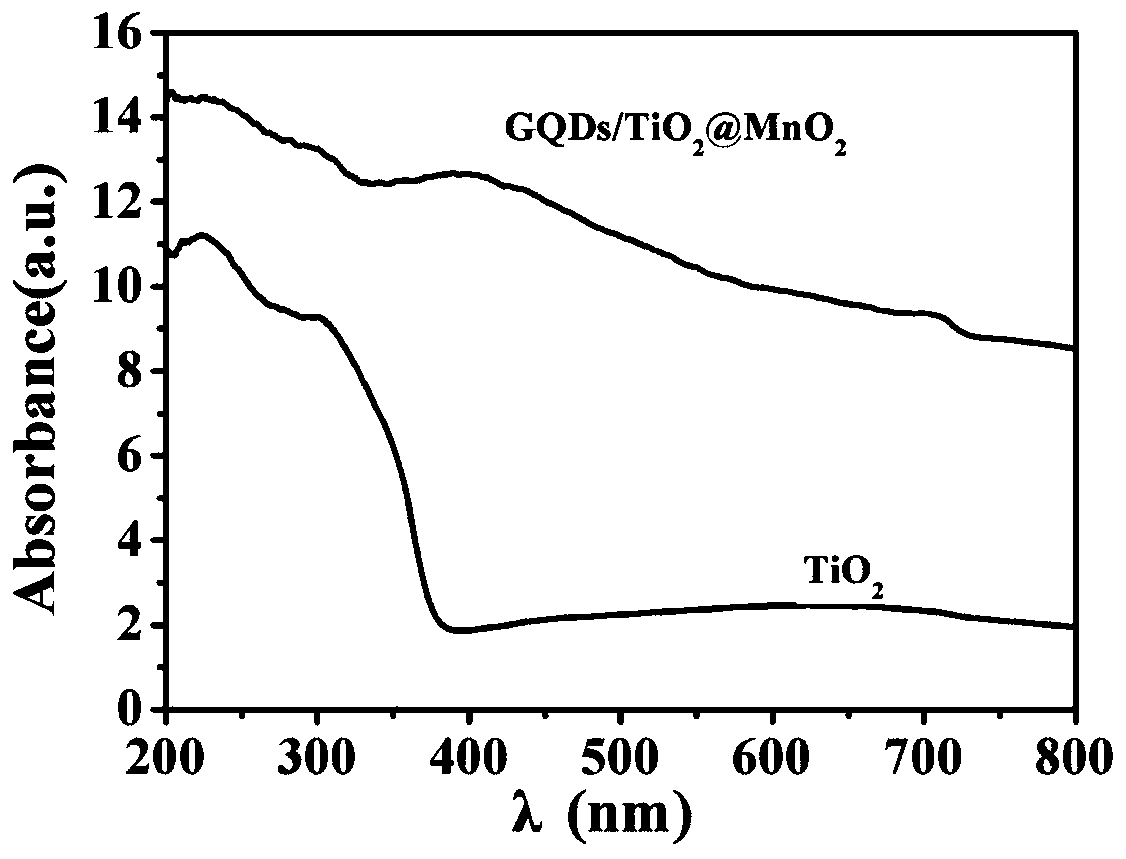
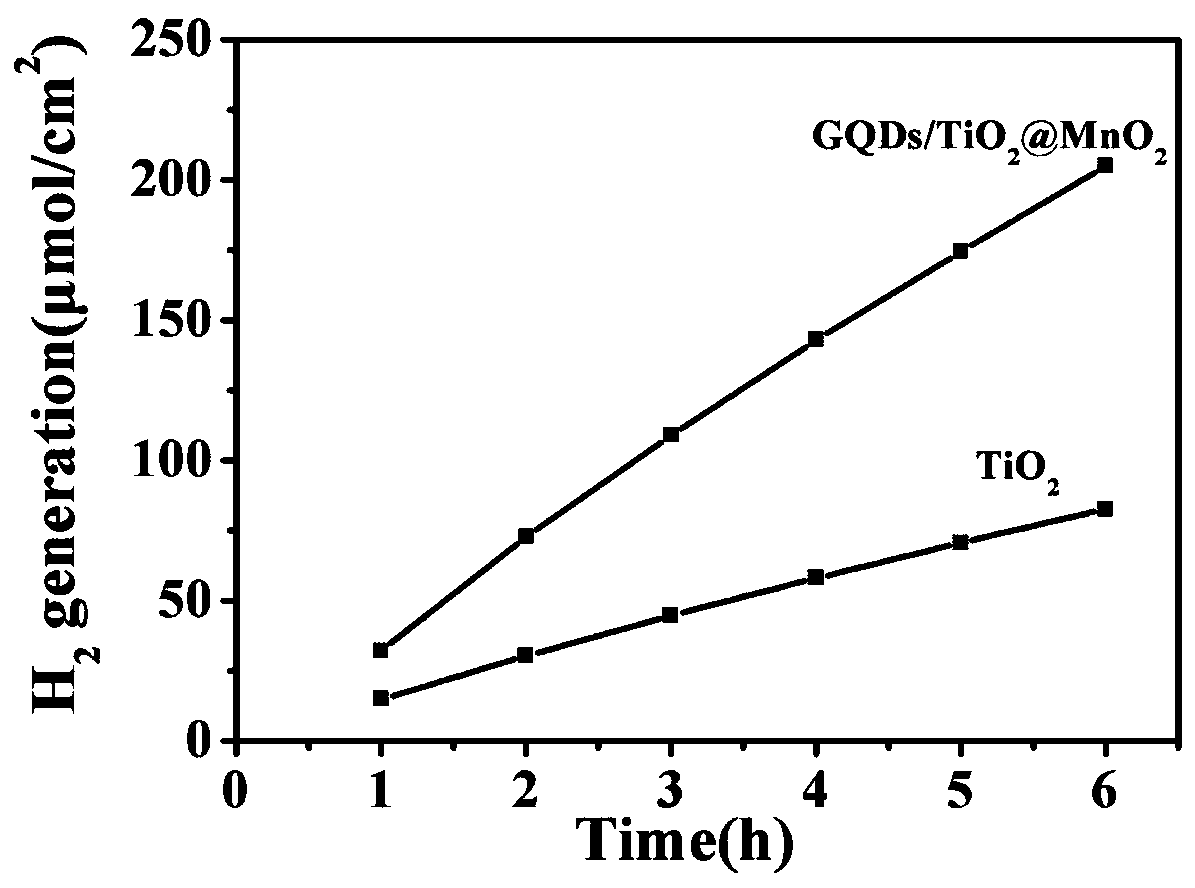
InactiveCN109794234ABroaden the absorbing boundaryAbsorption Boundary ExtendedHydrogen productionMetal/metal-oxides/metal-hydroxide catalystsWater bathsTio2 nanotube
The invention provides a graphene quantum dot modified manganese oxide / titanium oxide nanotube array material and a preparation method and application thereof, and belongs to the field of photocatalysis of film materials. A titanium dioxide material is composited and modified through graphene quantum dots and manganese oxide; compared with titanium dioxide materials, the titanium dioxide materialmodified with quantum dots have improved electrochemical properties; the compositing with manganese oxide enables absorbing boundary of titanium dioxide to be widened, forbidden band width of titaniumdioxide can be evidently decreased, the absorbing boundary is extended, titanium dioxide can utilize visible light more efficiently, compositing of photo-induced electrons and holes is inhibited, photocatalytic activity of titanium dioxide is improved, and hydrogen can be produced by photoelectric catalysis of titanium dioxide more efficiently; in addition, reduced graphene oxide and titanium sheeting are nontoxic and pollution free, the cost is low, and the preparation method has the advantages of simplicity, convenience and high speed; the morphology and properties are controlled by controlling reaction voltage and time and water bath reaction time, and the operation is simple.
Owner:HEFEI UNIV OF TECH
Preparation method of gold-iron oxyhydroxide-cuprous oxide-copper sulfide composite paper
ActiveCN111893503AEasy to separateSpeed up the transfer processElectrolytic inorganic material coatingLiquid/solution decomposition chemical coatingPtru catalystHigh volume manufacturing
The invention discloses a preparation method of gold-iron oxyhydroxide-cuprous oxide-copper sulfide composite paper. The preparation method comprises the following steps of: growing a layer of gold nanoparticles on the surface of a paper chip to obtain a gold paper electrode; sequentially depositing iron oxyhydroxide and cuprous oxide on the surface of the gold paper electrode by utilizing an electro-deposition method; and finally, coating the surface of the cuprous oxide with copper sulfide through a continuous ion layer adsorption and reaction method, so as to obtain the gold-iron oxyhydroxide-cuprous oxide-copper sulfide composite paper. The iron oxyhydroxide and copper sulfide are used as cocatalysts, so that separation and transfer of photogenerated charges of the cuprous oxide can beeffectively accelerated, the photostability of the cuprous oxide is enhanced, and the photocatalytic hydrogen production performance of the cuprous oxide is greatly improved. The composite paper is rich in raw material source, simple in preparation method and suitable for mass production, and has high application value in the field of photocatalytic hydrogen production.
Owner:UNIV OF JINAN
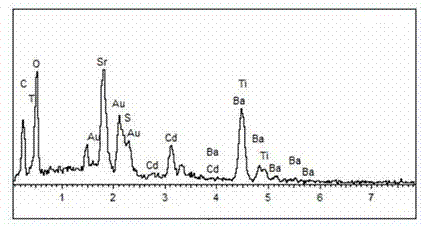
Material CdS/Ba0.4Sr0.6TiO3 for producing hydrogen by photocatalytically decomposing water and preparation method thereof
ActiveCN103586050AEasy to operateHigh purityPhysical/chemical process catalystsHydrogen productionCatalytic effectReagent
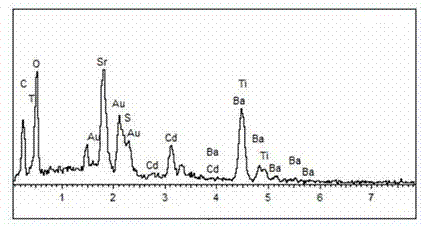
The invention relates to a material CdS / Ba0.4Sr0.6TiO3 for producing hydrogen by photocatalytically decomposing water, which is a heterostructure catalyst formed by supporting CdS onto Ba0.4Sr0.6TiO3. The catalyst is prepared by a one-step hydrothermal process. When the mole ratio of the CdS to the Ba0.4Sr0.6TiO3 is 0.4:1, the expression is 40%CdS / Ba0.4Sr0.6TiO3, and the material has the optimal catalytic effect. Under simulated sunlight, by using Na2S / Na2SO3 as a sacrifice reagent, the hydrogen production efficiency by photocatalytically decomposing water of the 40%CdS / Ba0.4Sr0.6TiO3 is up to 1268.4 mu mol.h<-1>.g<-1> under the condition of no noble metal for cocatalysis. The one-step hydrothermal process is directly used for synthesizing the catalyst, is simple to operate, has the advantages of low production cost, higher synthesis yield, higher purity and favorable repetitiveness, and is suitable for the requirement for scale-up production; the catalyst has high stability and is convenient for reutilization; and the catalyst has very high photocatalytic hydrogen production ratio under simulated sunlight.
Owner:NANCHANG HANGKONG UNIVERSITY
An anatase tio 2 Preparation method of crystal facet heterojunction
ActiveCN110227433BExtend your lifeImprove photocatalytic performancePhysical/chemical process catalystsHydrogen productionHeterojunctionComposite substrate
Owner:ZHEJIANG UNIV
Preparation method and application of molybdenum disulfide/boron-doped graphite-phase carbon nitride composite visible light photocatalyst
PendingCN112295584ASimple processLow costPhysical/chemical process catalystsHydrogen productionPhoto catalysisEfficient catalyst
The invention discloses a preparation method and application of a molybdenum disulfide / boron-doped graphite-phase carbon nitride composite visible light photocatalyst, wherein boron-doped graphite-phase carbon nitride is prepared by reacting and sintering urea and sodium tetraphenylborate (PH4BNa), and molybdenum disulfide is prepared by a hydrothermal method. According to the invention, boron-doped graphite-phase carbon nitride photocatalyst is compounded with proper molybdenum disulfide to prepare an efficient catalyst, wherein the boron-doped graphite-phase carbon nitride has high specificsurface area and chemical stability, and molybdenum disulfide can accelerate separation of photo-excited electron hole pairs in boron-doped graphite-phase carbon nitride, so that excellent catalytic activity is achieved, the photocatalytic hydrogen production efficiency of the molybdenum disulfide / boron-doped graphite-phase carbon nitride photocatalyst is improved, and the photocatalyst has potential application value in the aspect of coping with energy crisis.
Owner:NANCHANG HANGKONG UNIVERSITY
A single-atom photocatalyst for hydrogen production and its preparation method and application
ActiveCN112295574BImprove photocatalytic hydrogen production efficiencyHigh activityPhysical/chemical process catalystsHydrogen productionPtru catalystPorous sheet
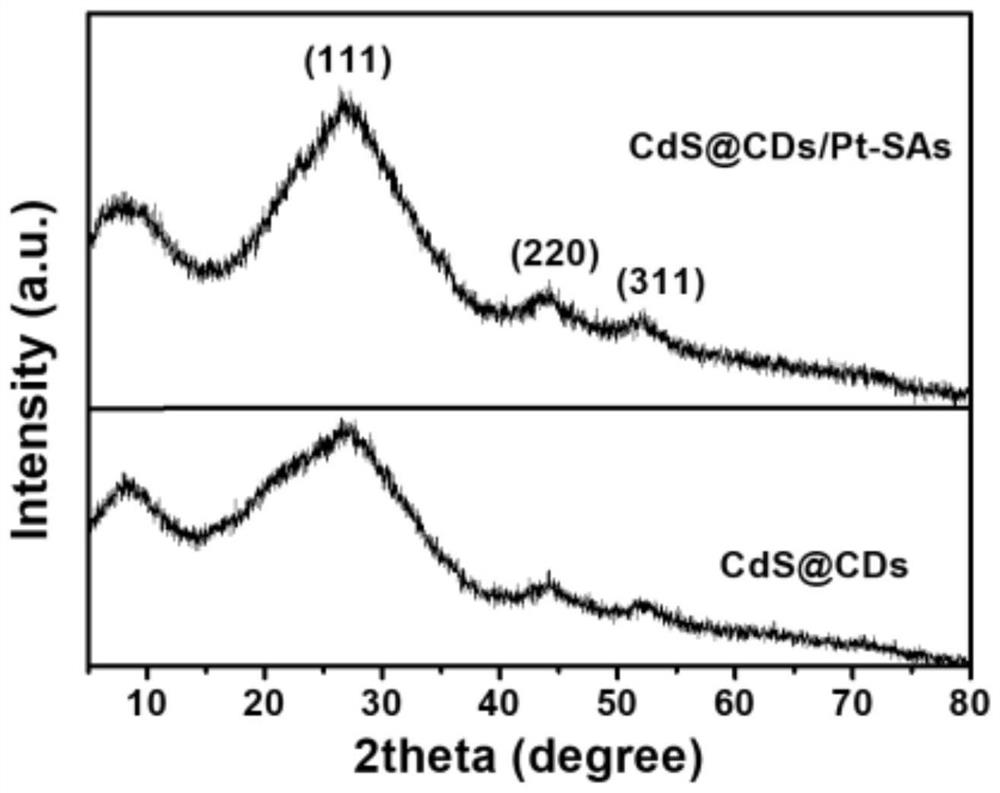
The present invention provides a novel single-atom photocatalyst for hydrogen production. The photocatalyst for hydrogen production has an ultra-thin porous sheet structure, and the Pt single atom is reduced in situ by abundant groups on the surface of carbon dots (CDs), and the obtained catalyst expresses The formula is CdS@CDs / Pt‑SAs. The photocatalyst for hydrogen production first synthesized cadmium acetate (Cd(CH 3 COO) 2 2H 2 O), n-butylamine (C 4 h 11 N) The ultra-thin Cd(OH) was synthesized in the reaction system of ethylene glycol 2 @CDs nanosheets and convert them into porous CdS@CDs nanosheets. Then, Pt single atoms (Pt‑SAs) were in situ reduced by CDs, and the anchoring of Pt‑SAs on CdS@CDs nanosheets was achieved. Pt single atoms have a strong ability to capture photogenerated electrons, leading to photocatalytic H 2 The evolution activity is remarkable, and its rate reaches 45.5mmol h ‑1 g ‑1 , which is 133 times that of CdS@CDs. The present invention opens up a way to prepare metal single atoms for catalyzing various useful reactions.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
A kind of silver nanoparticle/titanium dioxide nanoflower composite material and its preparation method and application
ActiveCN108499561BReduce manufacturing costSimple preparation processWater/sewage treatment by irradiationWater treatment compoundsPhotoelectric conversionTitanium oxide
The invention discloses a preparation method for depositing silver on the surface of titanium dioxide nanoflowers. The silver nanoparticle / titanium dioxide nanoflower composite material prepared by the invention is composed of titanium dioxide nanoflowers and silver nanoparticles, in which the titanium dioxide nanoflowers provide a large specific surface area. And rich in oxygen vacancies. Silver nanoparticles are uniformly reduced and deposited on the surface of titanium dioxide, and there is close interface contact between the two. The silver nanoparticle / titanium dioxide nanoflower composite material of the present invention is an efficient and stable photoelectric conversion material. It adopts a one-step simple reduction method, has a simple preparation process and easy control of reaction conditions, and is suitable for large-scale preparation and industrial production.
Owner:ZHEJIANG UNIV CITY COLLEGE
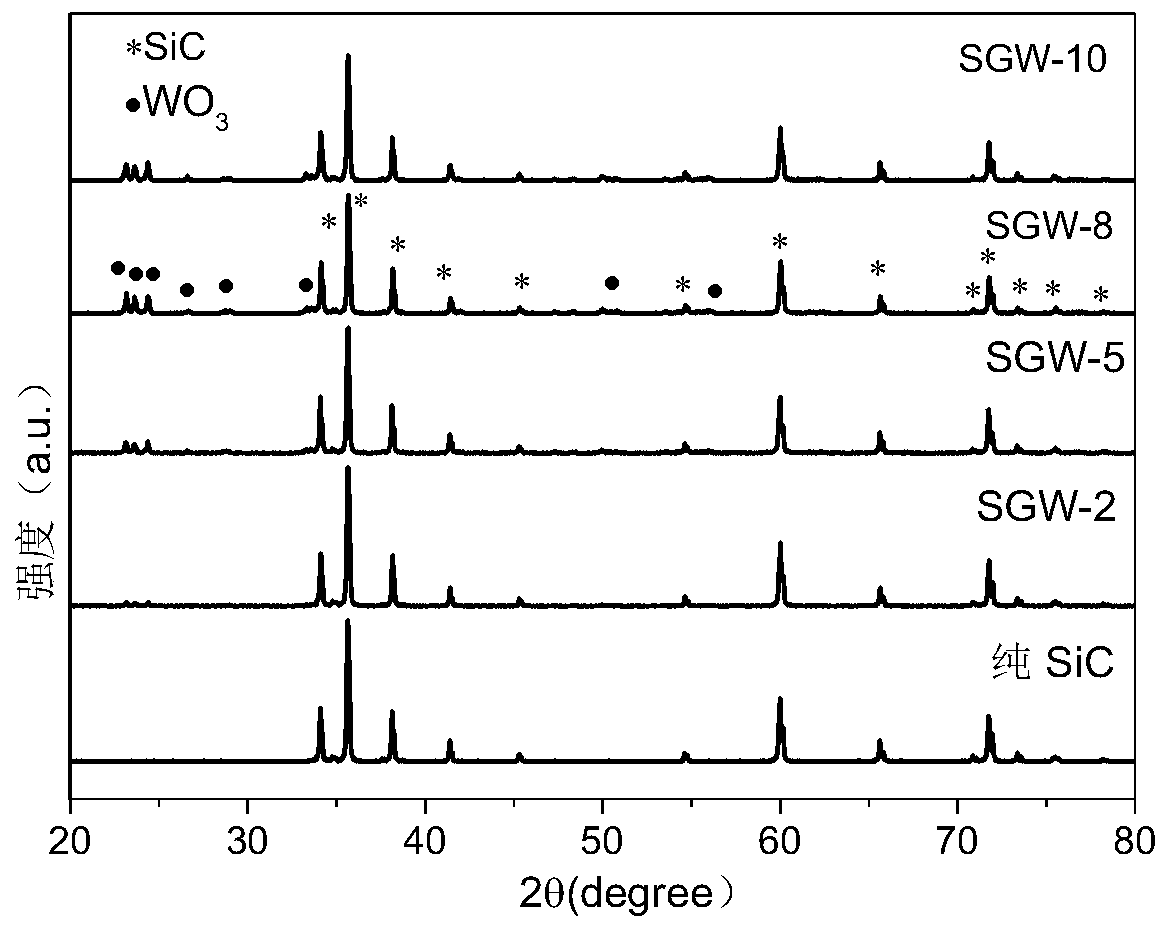
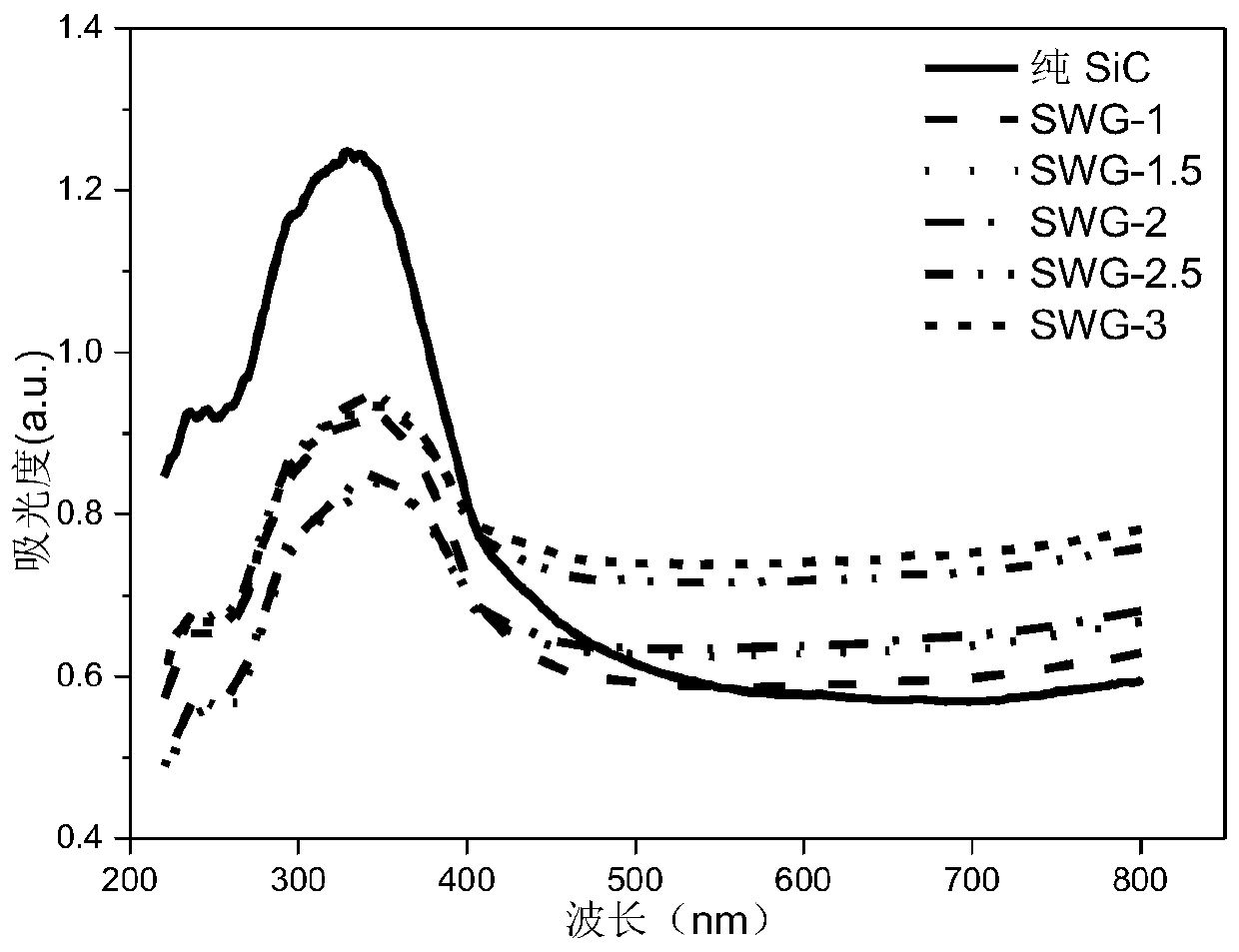
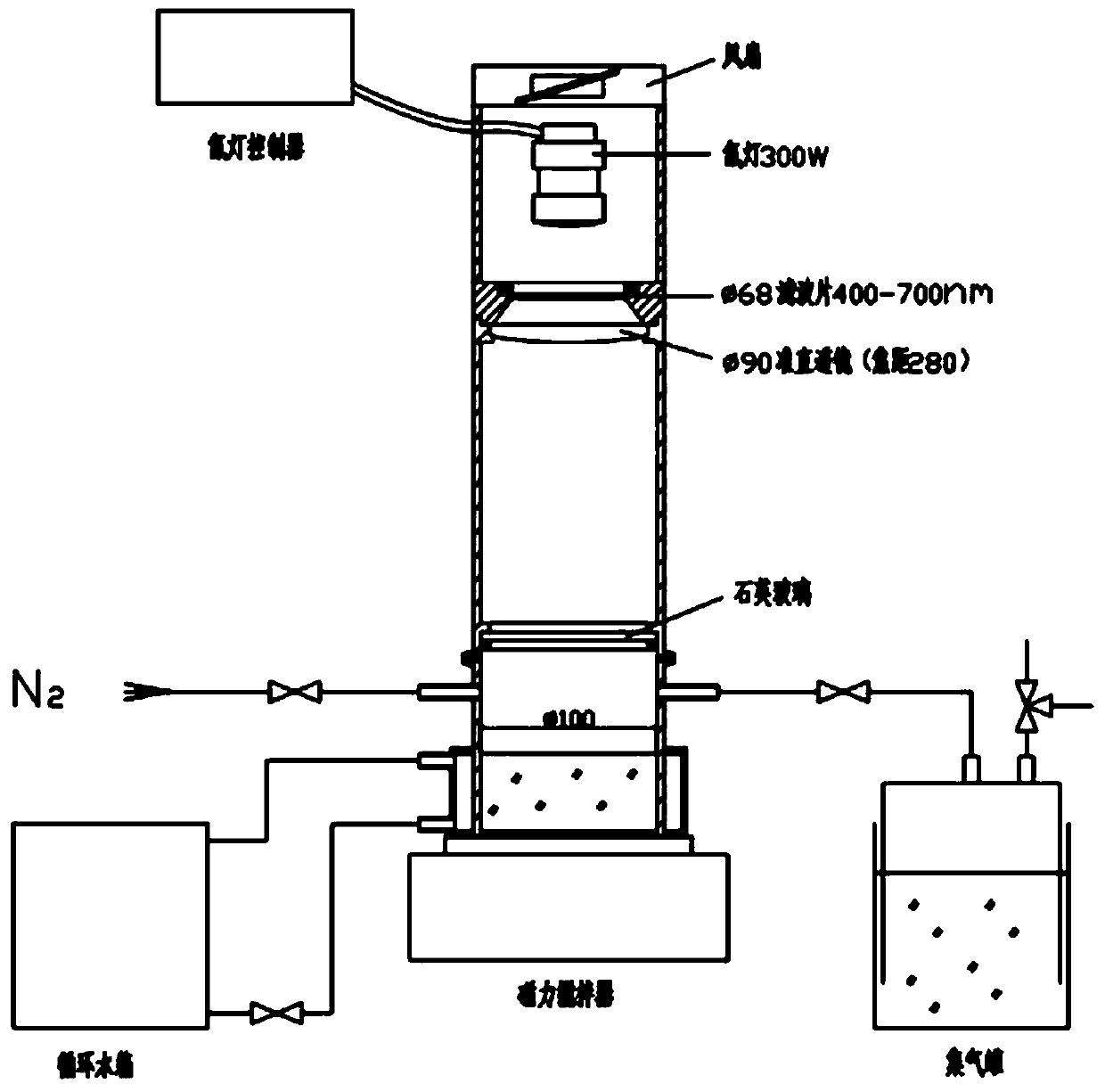
Preparation method and application of visible light response hydrogen generation photocatalyst GO/SiC/WO3
ActiveCN109926080AEasy to separateIncrease surface areaPhysical/chemical process catalystsHydrogen productionDecomposition1-Butyl-3-methylimidazolium hexafluorophosphate
Owner:SHANDONG UNIV OF SCI & TECH
Doped quantum dot catalyst and preparation method thereof, hydrogen production system and hydrogen production method comprising doped quantum dot catalyst
ActiveCN105478148BUniform sizeGood dispersionHydrogenPhysical/chemical process catalystsPhoto catalyticPtru catalyst
The invention discloses a quantum dot catalyst doped with metal ions. The catalyst includes a light-harvesting unit and a catalyzing unit, wherein the light-harvesting unit includes one, two or several kinds of quantum dots, and the catalyzing unit comprises the metal ions doped in the quantum dots. The metal ions are dispersed on the quantum dots in the following one or several manners that: (1) the metal ions are adhered to the surface of the quantum dots; (2) the metal ions are uniformly dispersed in the quantum dots; (3) the metal ions are disposed in the quantum dots in a gradient alloy way; (4) the metal ions are merely disposed on cores of core-shell quantum dots; (5) the metal ions are merely disposed on shells of the core-shell quantum dots; (6) both the cores and shells of the core-shell quantum dots are doped with the metal ions. The invention further discloses a photocatalytic hydrogen production system of doped quantum dots. The system has high efficiency of a quantum-dot-catalyst photocatalytic hydrogen production system and simplicity of a single quantum-dot photocatalytic hydrogen production system. The system can conveniently combine with an oxygen production half-reaction to totally decompose water.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Preparation method of a nitrogen-containing carbon quantum dot/graphite phase carbon nitride composite photocatalyst
ActiveCN107376967BOvercome the shortcomings of extraction and separation difficultiesImprove photocatalytic hydrogen production efficiencyPhysical/chemical process catalystsHydrogen productionSolventIonic liquid
The invention belongs to the technical field of photocatalytic composite materials, and particularly relates to a preparation method of a nitrogen-containing carbon quantum dot / graphite phase carbon nitride composite photocatalyst: using glucose and glycine as carbon precursors and nitrogen precursors, and using a high-speed ball milling method to synthesize Nitrogen-containing carbon quantum dots; graphite-phase carbon nitride is prepared by hydrothermal method; using hydrophilic ionic liquid as solvent, nitrogen-containing carbon quantum dots and graphite-phase carbon nitride are combined through an acylation reaction to prepare nitrogen-containing carbon Quantum dot / graphite phase carbon nitride composite photocatalyst, nitrogen-containing carbon quantum dots and graphite phase carbon nitride are combined by covalent bonds.
Owner:CHANGZHOU UNIV
Material CdS/Ba0.4Sr0.6TiO3 for producing hydrogen by photocatalytically decomposing water and preparation method thereof
ActiveCN103586050BEasy to operateHigh purityPhysical/chemical process catalystsHydrogen productionCatalytic effectReagent
The invention relates to a material CdS / Ba0.4Sr0.6TiO3 for producing hydrogen by photocatalytically decomposing water, which is a heterostructure catalyst formed by supporting CdS onto Ba0.4Sr0.6TiO3. The catalyst is prepared by a one-step hydrothermal process. When the mole ratio of the CdS to the Ba0.4Sr0.6TiO3 is 0.4:1, the expression is 40%CdS / Ba0.4Sr0.6TiO3, and the material has the optimal catalytic effect. Under simulated sunlight, by using Na2S / Na2SO3 as a sacrifice reagent, the hydrogen production efficiency by photocatalytically decomposing water of the 40%CdS / Ba0.4Sr0.6TiO3 is up to 1268.4 mu mol.h<-1>.g<-1> under the condition of no noble metal for cocatalysis. The one-step hydrothermal process is directly used for synthesizing the catalyst, is simple to operate, has the advantages of low production cost, higher synthesis yield, higher purity and favorable repetitiveness, and is suitable for the requirement for scale-up production; the catalyst has high stability and is convenient for reutilization; and the catalyst has very high photocatalytic hydrogen production ratio under simulated sunlight.
Owner:NANCHANG HANGKONG UNIVERSITY
Photocatalyst cd/cds containing cocatalyst cd and its preparation and application in photocatalytic hydrogen production
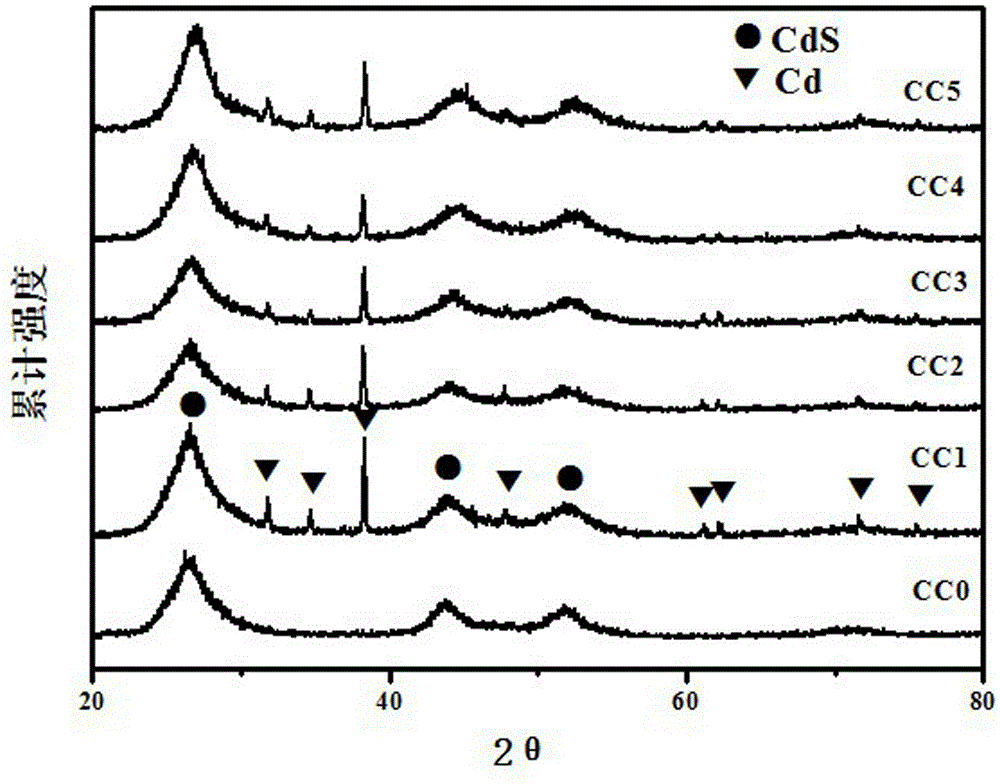
InactiveCN103316693BImprove photocatalytic hydrogen production efficiencyEfficient separationPhysical/chemical process catalystsHydrogen productionPhotocatalytic water splittingCadmium sulphide
The invention provides a photocatalyst Cd / CdS containing a cocatalyst Cd, which uniformly adheres elemental metal Cd on the surface of CdS, and belongs to the technical field of photocatalysis. The metal simple substance Cd introduced in the cadmium sulfide CdS of the present invention is used as a cocatalyst, which can effectively separate photogenerated electrons and hole pairs, greatly improves the photocatalytic hydrogen production efficiency of CdS, and expands the use of CdS in the photocatalytic water splitting hydrogen production reaction The application of high-efficiency photocatalyst Cd / CdS was directly synthesized by photochemical method, the structure and performance are relatively stable, and it can be recycled in the photocatalytic water splitting hydrogen production reaction. In addition, the synthesis method of the invention is simple, the cost is low, the yield is high, and the industrialization prospect is good.
Owner:NORTHWEST NORMAL UNIVERSITY
Preparation method of gold-iron oxyhydroxide-cuprous oxide-copper sulfide composite paper
ActiveCN111893503BEasy to separateSpeed up the transfer processElectrolytic inorganic material coatingLiquid/solution decomposition chemical coatingPtru catalystEngineering
The invention discloses a preparation method of gold-iron oxyhydroxide-cuprous oxide-copper sulfide composite paper. First, a layer of gold nanoparticles is grown on the surface of a paper chip to obtain a gold paper electrode; Iron oxyhydroxide and cuprous oxide were deposited on the surface in sequence; finally, copper sulfide was coated on the surface of cuprous oxide by continuous ion layer adsorption and reaction method to obtain gold-iron oxyhydroxide-cuprous oxide-copper sulfide composite paper. Iron oxyhydroxide and copper sulfide as cocatalysts can effectively accelerate the separation and transfer of photogenerated charges of cuprous oxide, thereby enhancing the photostability of cuprous oxide and greatly improving its photocatalytic hydrogen production performance. The composite paper has rich sources of raw materials, simple preparation method, is suitable for mass production, and has high application value in the field of photocatalytic hydrogen production.
Owner:UNIV OF JINAN
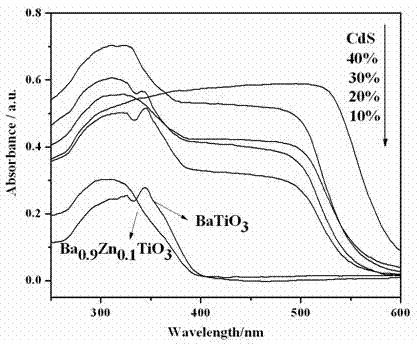
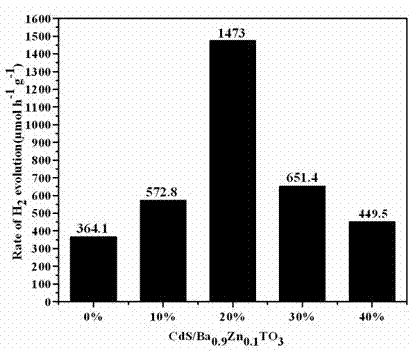
Photocatalytic water splitting hydrogen production material CdS/Ba0.9Zn0.1TiO3 and preparation method thereof
InactiveCN103381367BEasy to operateHigh purityPhysical/chemical process catalystsHydrogen productionPhotocatalytic water splittingCatalytic effect
A photocatalytic water splitting hydrogen production material CdS / Ba0.9Zn0.1TiO3 is a catalyst with a heterostructure formed by enabling CdS to be loaded to Ba0.9Zn0.1TiO3 , and the catalyst is obtained by a hydrothermal sol-gel process. When the molar ratio of CdS and Ba0.9Zn0.1TiO3 is 1:5, the expression is 20%CdS / Ba0.9Zn0.1TiO3 , and the catalytic effect of the material is optimal. Under the exposure of a xenon lamp of 300 watts, Na2S / Na2SO3 serves as a sacrifice reagent. The photocatalytic water splitting hydrogen production material CdS / Ba0.9Zn0.1TiO3 has the advantages that the catalyst is directly synthesized in a hydrothermal sol-gel process and is simple in operate, low in production cost, high in synthesis yield, extremely high in purity, good in repeatability and suitable for the requirements of expanded production; the catalyst is good in stability and convenient to recycle; and the catalyst is high in photocatalysis hydrogen production efficiency.
Owner:NANCHANG HANGKONG UNIVERSITY
A kind of graphite phase carbon nitride foam photocatalytic material and preparation method thereof
ActiveCN109261191BEasy to makeWide variety of sourcesPhysical/chemical process catalystsHydrogen productionFreeze-dryingSlurry
The invention relates to a graphite-phase carbon nitride foam photocatalyst material and a preparation method thereof. Its technical scheme is: add 5-14 mass parts of graphite phase carbon nitride into 100 mass parts of deionized water, mix, then add 1.7-2.0 mass parts of sodium dodecylsulfonate, 1.7-2.0 mass parts Dodecyl alcohol and 1.7 to 2.0 parts by mass of resin glue are stirred at 40 to 60°C and 100 to 200r / min for 10 to 20 minutes to obtain a mixed solution; then the mixed solution is stirred at 1500 to 2000r / min 15 to 20 minutes, then add 5 to 14 parts by mass of binder, and continue to stir for 5 to 10 minutes to obtain a graphite-phase carbon nitride foam slurry; cast the graphite-phase carbon nitride foam slurry and freeze-dry for 6 to 12 hours , drying at 80-100° C. for 18-24 hours to obtain a graphite-phase carbon nitride foam photocatalyst material. The invention has simple process and low cost; the photocatalytic hydrogen production efficiency of the prepared graphite-phase carbon nitride foam photocatalytic material is high.
Owner:WUHAN UNIV OF SCI & TECH
A method for preparing zinc oxide/zinc sulfide nano-heterojunction photocatalyst
ActiveCN106622291BLarge-scale synthesisImprove photocatalytic hydrogen production efficiencyPhysical/chemical process catalystsHeterojunctionTalc / Zinc Oxide
A method for preparing zinc oxide / zinc sulfide nano-heterojunction photocatalyst. The catalyst is a heterojunction catalyst formed by loading ZnO onto ZnS. It is a two-step calcination method using MOF-5 as a template. When ZnS- When C is calcined in air for x minutes, its expression is ZnOS‑x. The advantages of the present invention are: 1. For the first time, a metal-organic framework complex is used as a template to prepare a heterojunction. Compared with the existing technology, the catalyst prepared by this method can reach the nanometer level and can be synthesized on a large scale; 2. The present invention The catalyst has high photocatalytic hydrogen production efficiency under visible light irradiation and does not require any co-catalyst; 3. The catalyst of the present invention has good stability and is easy to be reused.
Owner:NANKAI UNIV
Photocatalytic water-splitting hydrogen production material CdS/Sr1.6Zn0.4Nb2O7 and preparation method thereof
ActiveCN103521244BEasy to operateHigh purityPhysical/chemical process catalystsHydrogen productionHeterojunctionPhotocatalytic water splitting
A photocatalytic decomposing water hydrogen production material CdS / Sr1.6Zn0.4Nb2O7 is a catalyst having a heterojunction structure formed by loading CdS on Sr1.6Zn0.4Nb2O7. The inventive catalyst is prepared by a hydrothermal method through sol gel. When a molar ratio of the CdS and the Sr1.6Zn0.4Nb2O7 is 3:7, the expression is 30%CdS / Sr1.6Zn0.4Nb2O7, and the catalytic effect of the material is best. Under the illumination of a 300 watt xenon lamp, Na2S / Na2SO3 is taken as a sacrifice reagent, the inventive material 30%CdS / Sr1.6Zn0.4Nb2O7 photocatalyzes and decomposes the water to produce the hydrogen without a co-catalyst of noble metal, and the efficiency is 645.7 mu mol.h<-1>.g<-1>. The photocatalytic decomposing water hydrogen production material of the invention has the advantages that: 1 the inventive catalyst is directly synthesized by the hydrothermal method through sol gel, the operation is simple, the production cost is low, the synthetic yield is higher, the purity is high and the repeatability is good, and the inventive catalyst is suitable for the demand of magnification production; 2, the inventive catalyst is good in the stability and is easy to reuse; and 3, the inventive catalyst has higher photocatalytic hydrogen production efficiency.
Owner:NANCHANG HANGKONG UNIVERSITY
A kind of gold nanoparticle/titanium dioxide nanoflower composite material and its preparation method and application
ActiveCN108579738BSimple preparation processFast transferHydrogen productionMetal/metal-oxides/metal-hydroxide catalystsNanoparticleOxygen vacancy
The invention discloses a method for preparing a gold nanoparticle / titanium dioxide nanoflower composite material. The gold nanoparticle / titanium dioxide nanoflower composite material prepared by the invention is composed of titanium dioxide nanoflowers and gold nanoparticles, in which the titanium dioxide nanoflowers provide large Specific surface area and rich in oxygen vacancies. Gold nanoparticles are uniformly deposited on the surface of titanium dioxide, forming a close contact interface between the two. The gold nanoparticle / titanium dioxide nanoflower composite material of the present invention is an efficient and stable photoelectric conversion material. It is prepared by a one-step simple reduction method. The preparation process is simple, the reaction conditions are easy to control, and it is suitable for large-scale preparation and industrial production.
Owner:ZHEJIANG UNIV CITY COLLEGE
A kind of titanium dioxide-platinum-cobalt tetraoxide ternary composite photocatalytic material and preparation method thereof
ActiveCN105664969BReduce hole recombination rateTo achieve the function of automatic separationHydrogen productionMetal/metal-oxides/metal-hydroxide catalystsElectron holePlatinum
The invention relates to a titanium dioxide-platinum-cobalt tetroxide ternary composite photocatalytic material and a preparation method thereof. Titanium dioxide-platinum-cobalt tetroxide ternary composite photocatalytic material uses titanium dioxide nanosheets with exposed {001} and {101} surfaces as carriers, platinum and cobalt tetroxide nanoparticles as co-catalysts, and platinum particles are loaded on {101} of titanium dioxide nanosheets. On the surface, cobalt tetroxide nanoparticles are supported on the {001} surface of titanium dioxide nanosheets. Its photocatalytic water decomposition efficiency is high, and the co-catalyst is closely combined with titanium dioxide, and has good long-term stability. It effectively solves the problem in the existing technology that photocatalyst electron holes are easily recombined, resulting in reduced photocatalytic efficiency.
Owner:WUHAN UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com