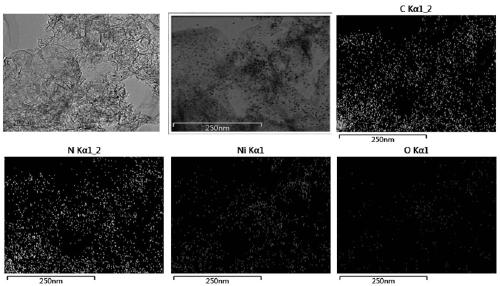
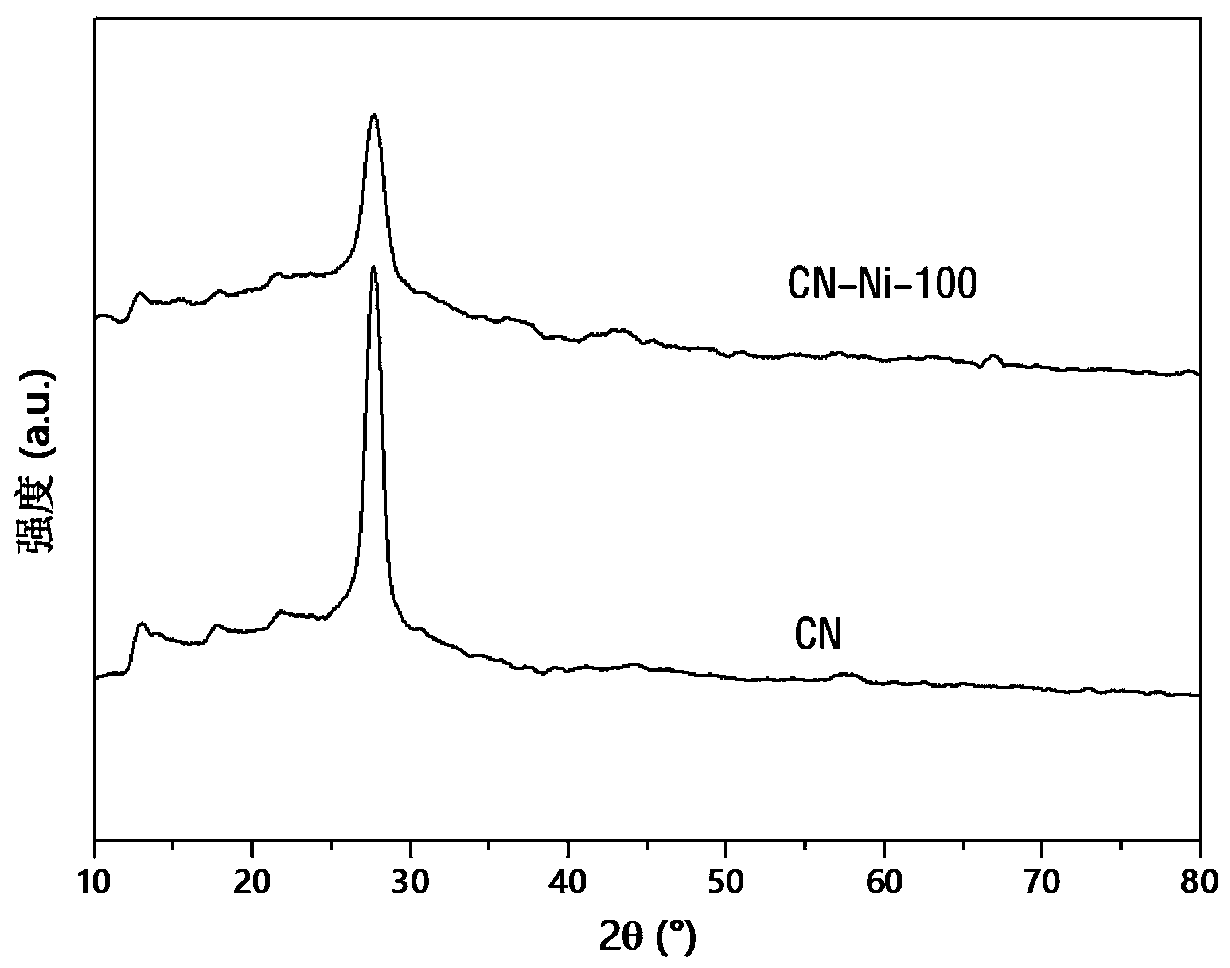
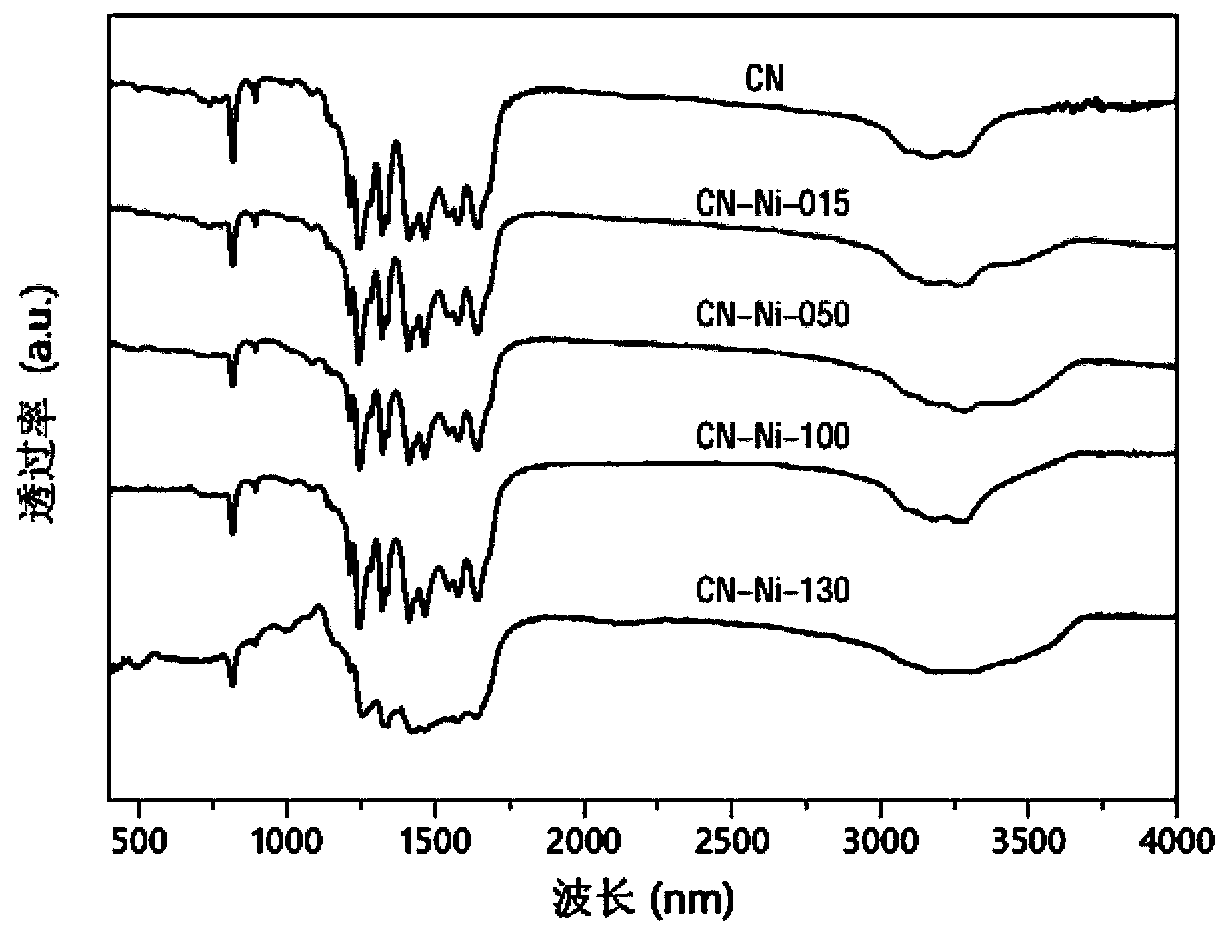
Nickel single-active site graphite-phase carbon nitride-based photocatalytic material as well as preparation method and application thereof
A technology of graphitic carbon nitride and photocatalytic materials, applied in chemical instruments and methods, physical/chemical process catalysts, inorganic chemistry, etc. Promote catalytic ability and other issues to achieve the effect of reducing production costs, improving efficiency, and improving the response to visible light
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Mix 10g urea with 100mg NiCl 2 ·6H 2 O was added to 50ml of deionized water, and stirred at room temperature to completely dissolve the two to form a mixed solution. Next, the above mixed solution was evaporated and concentrated to 10 ml at a constant temperature of 70° C., and then placed in liquid nitrogen and quickly frozen into ice. After the mixed solution ice is further pulverized and finely ground, it is placed in a freeze dryer to dry overnight, and excess solvent is removed to obtain the mixed precursor powder. The obtained precursor powder was transferred to a corundum crucible, and calcined at 550°C for 2 hours to obtain a nickel single active site graphite phase carbon nitride-based photocatalytic material, which was designated as CN-Ni-100;
[0041] Through nitrogen adsorption-desorption experiments, the specific surface area of CN-Ni-100 was measured to be 172.9m 2 / g;
[0042] Add 50 mg of the obtained sample to a mixed solution of 100 ml of water a...
Embodiment 2
[0044] Mix 10g urea with 50mg NiCl 2 ·6H 2 O was added to 50ml of deionized water, and stirred at room temperature to completely dissolve the two to form a mixed solution. Next, the above mixed solution was evaporated and concentrated to 10 ml at a constant temperature of 70° C., and then placed in liquid nitrogen and quickly frozen into ice. After the mixed solution ice is further pulverized and finely ground, it is placed in a freeze dryer to dry overnight, and excess solvent is removed to obtain the mixed precursor powder. The obtained precursor powder was transferred to a corundum crucible, and calcined at 550°C for 2 hours to obtain a nickel single active site graphite phase carbon nitride-based photocatalytic material, which was designated as CN-Ni-050;
[0045] Through nitrogen adsorption-desorption experiments, the specific surface area of CN-Ni-50 was measured to be 144.4m 2 / g;
[0046] Add 50 mg of the obtained sample to a mixed solution of 100 ml of water and...
Embodiment 3
[0048] Mix 10g urea with 130mg NiCl 2 ·6H 2 O was added to 50ml of deionized water, and stirred at room temperature to completely dissolve the two to form a mixed solution. Next, the above mixed solution was evaporated and concentrated to 10 ml at a constant temperature of 70° C., and then placed in liquid nitrogen and quickly frozen into ice. After the mixed solution ice is further pulverized and finely ground, it is placed in a freeze dryer to dry overnight, and excess solvent is removed to obtain the mixed precursor powder. The obtained precursor powder was transferred to a corundum crucible, and calcined at 550°C for 2 hours to obtain a nickel single active site graphite phase carbon nitride-based photocatalytic material, which was designated as CN-Ni-130;
[0049] Through nitrogen adsorption-desorption experiments, the specific surface area of CN-Ni-130 was measured to be 102.6m 2 / g;
[0050] Add 50 mg of the obtained sample to a mixed solution of 100 ml of water a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com