Preparation method and application of molybdenum disulfide/boron-doped graphite-phase carbon nitride composite visible light photocatalyst
A graphite-phase carbon nitride and molybdenum disulfide technology, applied in physical/chemical process catalysts, chemical instruments and methods, hydrogen/synthesis gas production, etc., can solve application limitations, low efficiency of photocatalytic water splitting, and charge separation efficiency Low-level problems, to achieve the effect of low cost, less impurities, and complete reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] Step 1: Prepare boron-doped graphite phase carbon nitride powder: weigh 20 g urea, 12 mg PH 4 BNa and 30 mL of water. Put the weighed medicine into a beaker, put it into an ultrasonic disperser and sonicate it for 30 min, then move it to a magnetic stirrer, adjust to a suitable position, and stir at room temperature for 30 min. Then heated in a constant temperature water bath set at 80 °C for 3 h, and evaporated to dryness to obtain a white powder. Put the white powder in a muffle furnace, raise the temperature to 520 °C, set the heating rate to 5 °C / min, and maintain this temperature for 2 h for sintering to obtain boron-doped graphite phase carbon nitride powder.
[0020] Step 2: Prepare molybdenum disulfide powder: 5.3142 g (NH 4 ) 6 Mo 7 o 24 4H 2 O and 9.8195 g of thiourea were vigorously stirred in 150 ml of distilled water. After the formation of a homogeneous solution, it was transferred to a 100 ml hydrothermal kettle and kept at 180°C for 24 hours, and ...
example 2
[0022] Example 2: The difference from Example 1 is that molybdenum disulfide is not added, and a boron-doped graphite phase carbon nitride catalyst sample is prepared.
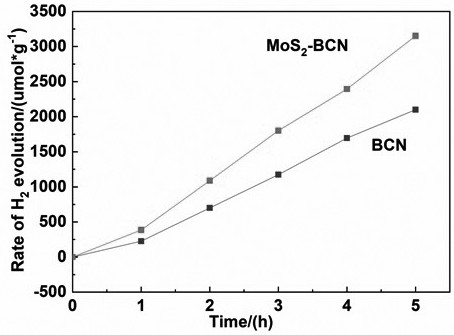
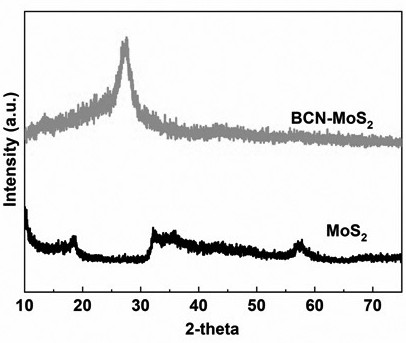
[0023] figure 1 For the photocatalytic hydrogen production average rate figure of the photocatalyst that example 1 and example 2 obtain, figure 2 It is example 1 and the XRD spectrum of molybdenum disulfide, as can be seen from the figure, the present invention obviously improves the hydrogen production of boron-doped graphite phase carbon nitride material through the composite of boron-doped graphite phase carbon nitride and molybdenum disulfide s efficiency.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com