Applications of five-ring triterpenoid compounds as oxidized squalene cyclase inhibitors
A technology of pentacyclic triterpenoids and oxidized dogfish, which is applied in the field of medicine and can solve the problems of toxicity, side effects, formation and malformation of lens epithelial cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
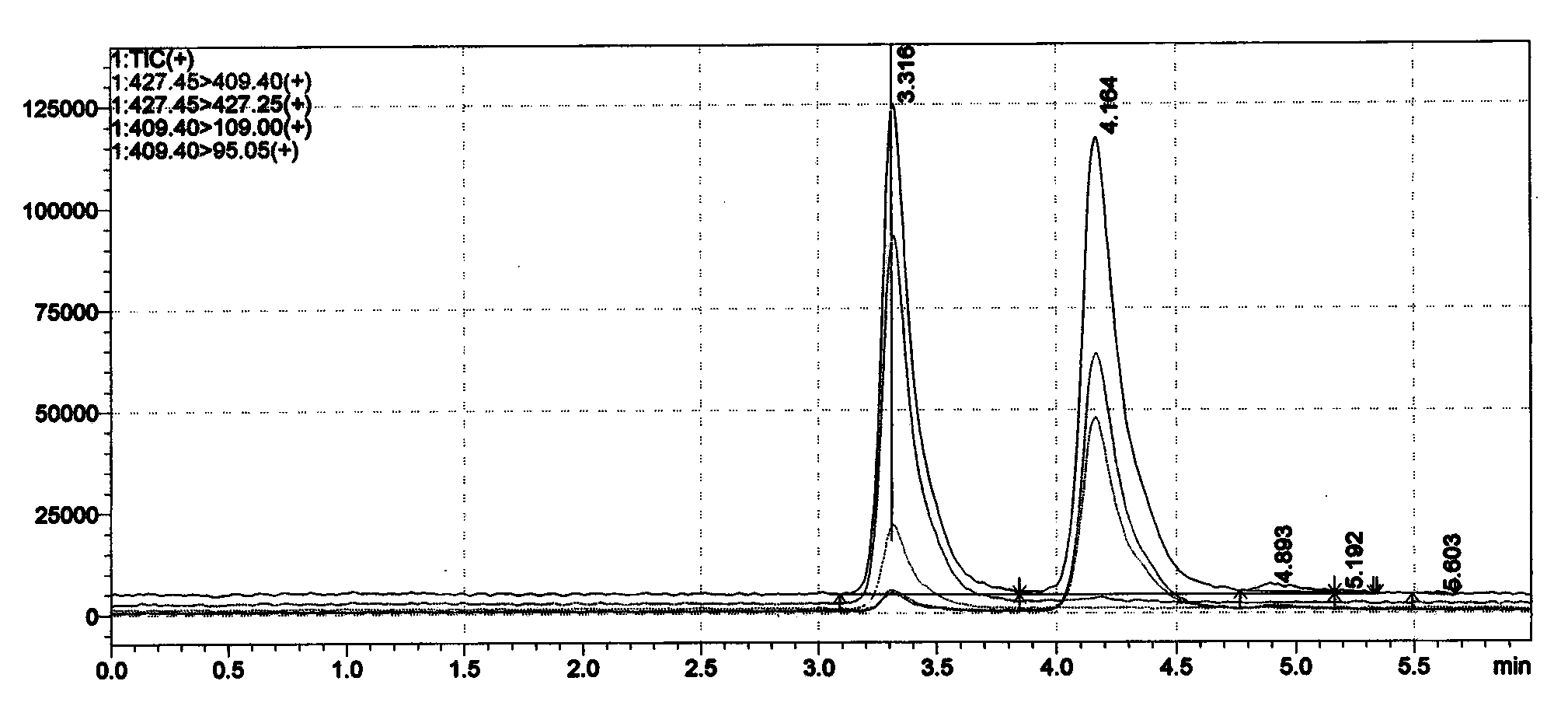
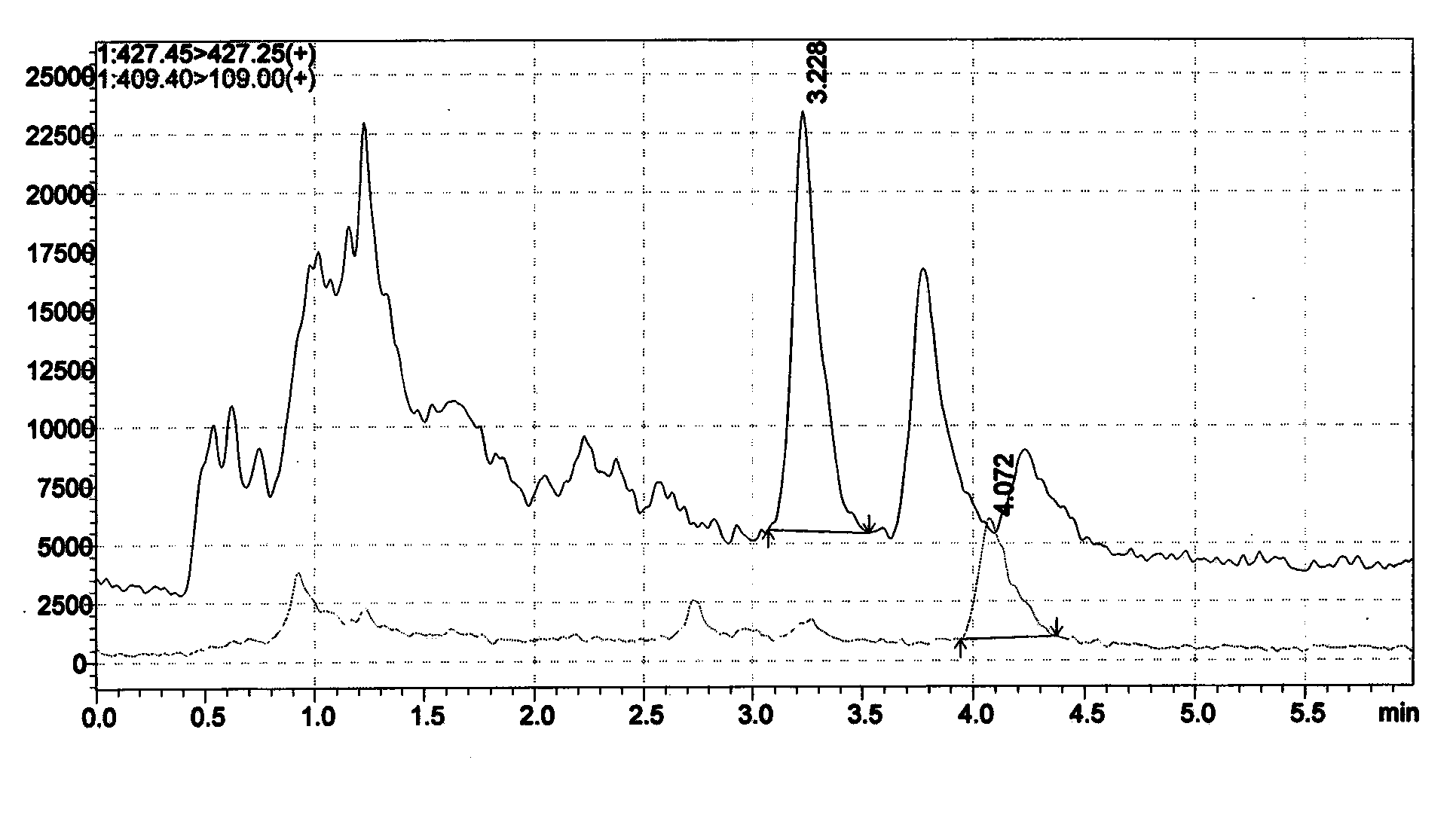
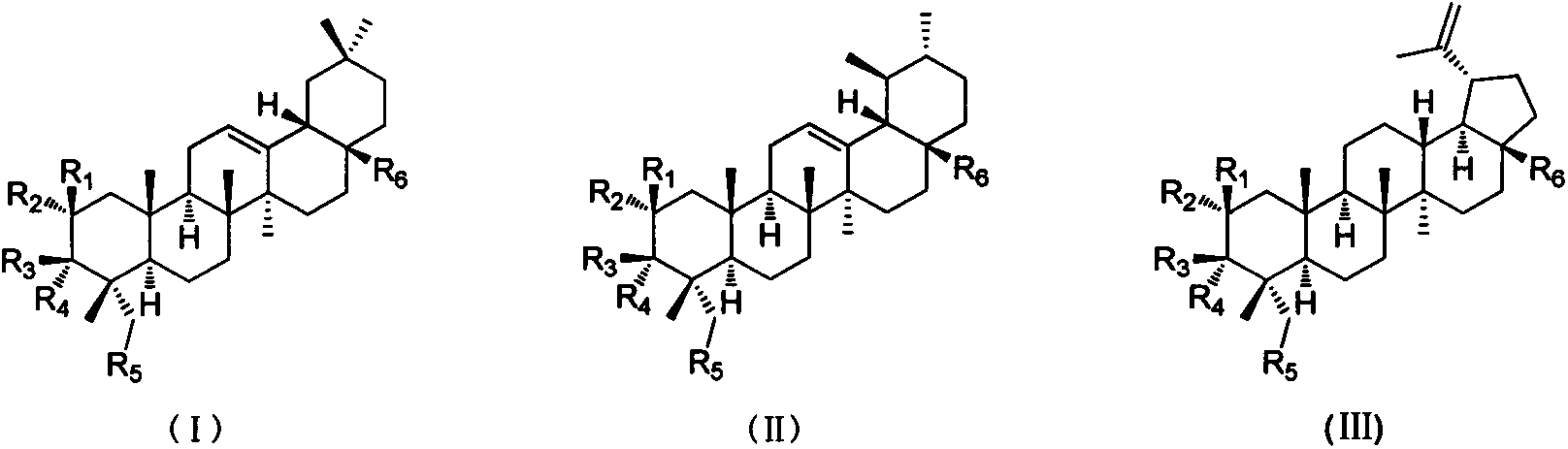
Image
Examples
Embodiment 1
[0039] Blood lipid lowering effect of commercially available oleanolic acid tablets on patients with hyperlipidemia
[0040] Method: 30 patients with hyperlipidemia were subjected to a clinical trial of lowering blood lipid of commercially available oleanolic acid tablets in patients with hyperlipidemia. None of the subjects used any lipid-lowering drugs or related health care products. The subjects took oleanolic acid tablets orally after meals every day, 3 times a day, 4 tablets at a time, 20mg / tablet, administered 7 days a week, for 1 week continuously. 24 hours after the last administration, fast overnight, take blood, take appropriate amount of blood, routinely prepare serum, observe blood lipid related indicators: serum total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL -C) and low-density lipoprotein cholesterol (LDL-C).
[0041] Results: Table 1 shows the effect of oleanolic acid tablets on lowering blood lipids of 3 hyperlipidemia p...
Embodiment 2
[0046] Effects of commercially available oleanolic acid tablets on relevant physiological indicators in patients with hyperlipidemia
[0047] Method: 30 patients with hyperlipidemia were subjected to a clinical trial of lowering blood lipid of commercially available oleanolic acid tablets in patients with hyperlipidemia. None of the subjects used any lipid-lowering drugs or related health care products. The subjects took oleanolic acid tablets orally after meals every day, 3 times a day, 4 tablets at a time, 20mg / tablet, administered 7 days a week, for 1 week continuously. 24 hours after the last administration, fast overnight, take blood, take appropriate amount of blood, routinely prepare serum, observe relevant physiological indicators: alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutamyl transpeptidase (GGT) ), creatine kinase (CK), urea (BUN), creatinine (Cr), uric acid (UA), C-reactive protein (CRP) and hypersensitive C-reactive protein (HsCRP).
...
Embodiment 3
[0053] Lipid-lowering effects of oleanolic acid, 3-acetyloleanolic acid, ursolic acid, and 3-acetylursolic acid on hyperlipidemic rats
[0054] Method: SD rats (200-220g, male) were adaptively fed for 1 week, and randomly divided into 2 groups: 6 normal control groups fed with standard diet and 36 model groups fed with high-fat diet. No intervention factors were applied to the normal control group. The model group was fed with high-fat diet (2% cholesterol, 0.5% sodium cholate, 3% lard, 0.2% propylthiouracil, 94.3% basic powder feed) for 2 weeks to form a high-fat model, and then randomly divided into vehicle group (6 rats) (fed with vehicle), atorvastatin calcium group (6 rats), oleanolic acid group (6 rats), 3-acetyl oleanolic acid group (6 rats), ursolic acid group ( 6) and 3-acetylursolic acid group (6). In addition to the normal control group, there were 7 groups in total. Among them, the atorvastatin calcium group was fed with 5 mg / kg / d of atorvastatin calcium, the ol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com