Blood perfusion device having anti-coagulation function and controlled-release function and manufacturing method thereof
A blood perfusion device and perfusion device technology, applied in the field of medical devices, can solve the problems of changing the injection dosage of anticoagulants, increasing the risk of pyrogen reaction, poor convenience, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
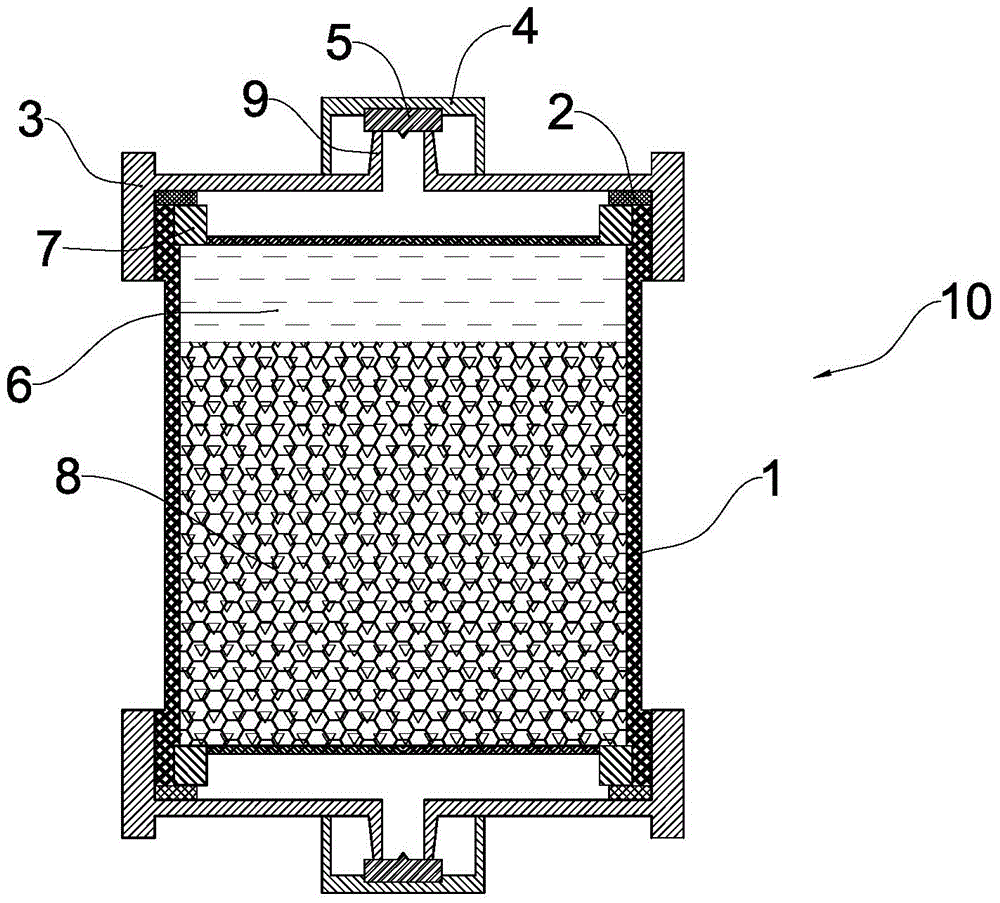
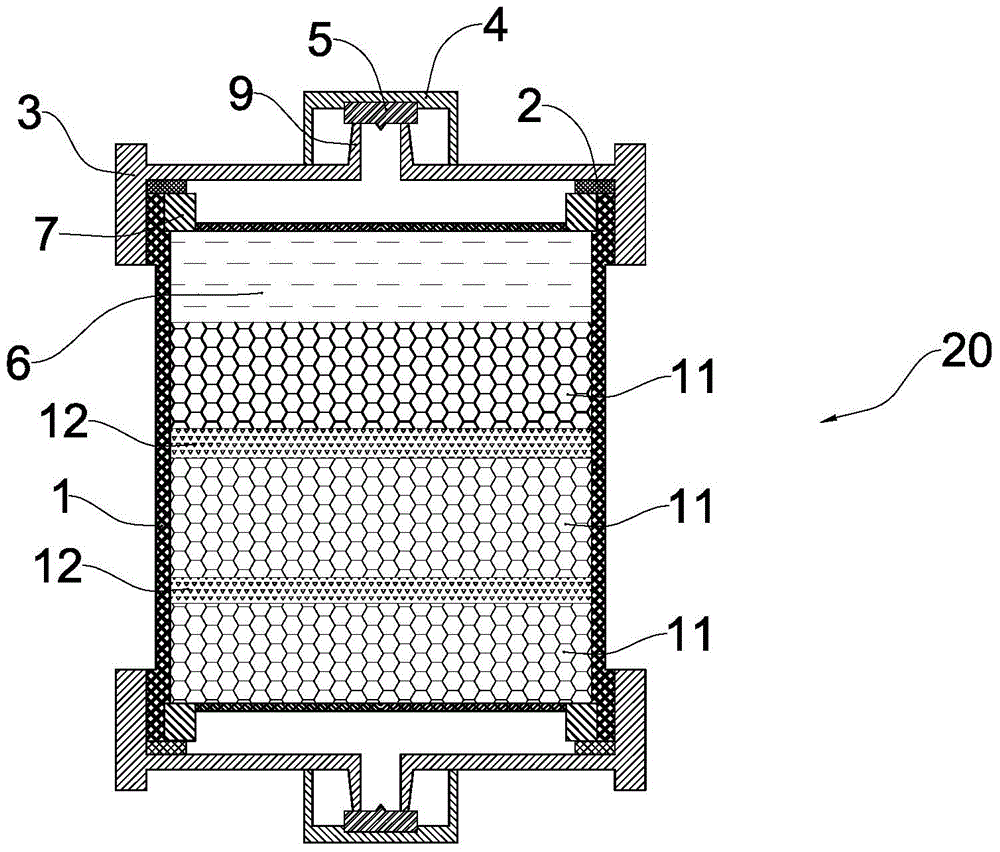
[0027] The preparation method of the above-mentioned hemoperfusion device includes providing such as figure 1 The shown perfusion device body and adsorbent, the perfusion device body has an adsorption chamber, and further includes the following steps: a) providing an anticoagulant slow-release gel with temperature-sensitive properties; b) coagulating the anticoagulant slow-release gel Filling the glue and the adsorbent into the adsorption chamber of the perfusate body; c) adding a preservation solution into the adsorption chamber; d) assembling the perfusate body.
[0028] Wherein, the preparation method of described anticoagulant slow-release gel comprises the steps:
[0029] 1) The anticoagulant, the compound containing double bonds at both ends (as a chemical crosslinking agent) and N-alkylacrylamide monomers are 5%~30%, 10%~15%, 60%~ Add 80% to sterile water for injection in turn to prepare an aqueous solution with a mass concentration of 10% to 50%. In a sealed container...
Embodiment 1
[0034] Add 50g of heparin sodium, 25g of methylenebisacrylamide, and 135g of N-isopropylacrylamide into 1L of sterile water for injection in sequence, and in a sealed container at 10°C, blow in nitrogen gas and stir for 60 minutes until the formation is complete. Dispersed homogeneous mixed aqueous solution; cool the above mixed aqueous solution to 1°C, add 1.5g of potassium persulfate after 30 minutes, inject 5mL of N,N,N',N'-tetramethylethylenediamine after 10 minutes, and protect with nitrogen Stirring was continued for 15 minutes; the stirred reaction solution was immediately poured into the forming mold, sealed and left to react at 18°C for 40 hours, and then soaked in 5% heparin sodium injection aqueous solution for 8 hours. Then the reaction product was rinsed with water for injection for 12 hours to obtain an anticoagulant sustained-release gel. The slow-release gel is transparent to milky white in appearance, with a volume of 0.05cm 3 . Then, 30 g of anticoagulant...
Embodiment 2
[0037] Add 10g of heparin calcium, 5g of ethylene glycol dimethacrylate, and 27g of N-isopropylacrylamide into 200mL of sterilized water for injection in sequence, and in a sealed container at 10°C, blow nitrogen into it and stir for 60 minutes until A completely dispersed uniform mixed aqueous solution is formed; the above mixed aqueous solution is cooled to 1°C, 0.36 g of ammonium persulfate is added after 30 minutes, and 1 mL of N,N,N',N'-tetramethylethylenediamine is injected after 10 minutes. Under the protection of nitrogen, continue to stir for 15 minutes; pour the stirred reaction solution into the molding mold immediately, seal it and let it stand for reaction at 18°C for 40 hours, and then soak it in the aqueous solution of heparin calcium injection with a mass fraction of 40%. Hours, and then the reaction product was rinsed with water for injection for 12 hours to obtain an anticoagulant sustained-release gel. The slow-release gel is transparent to milky white in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com