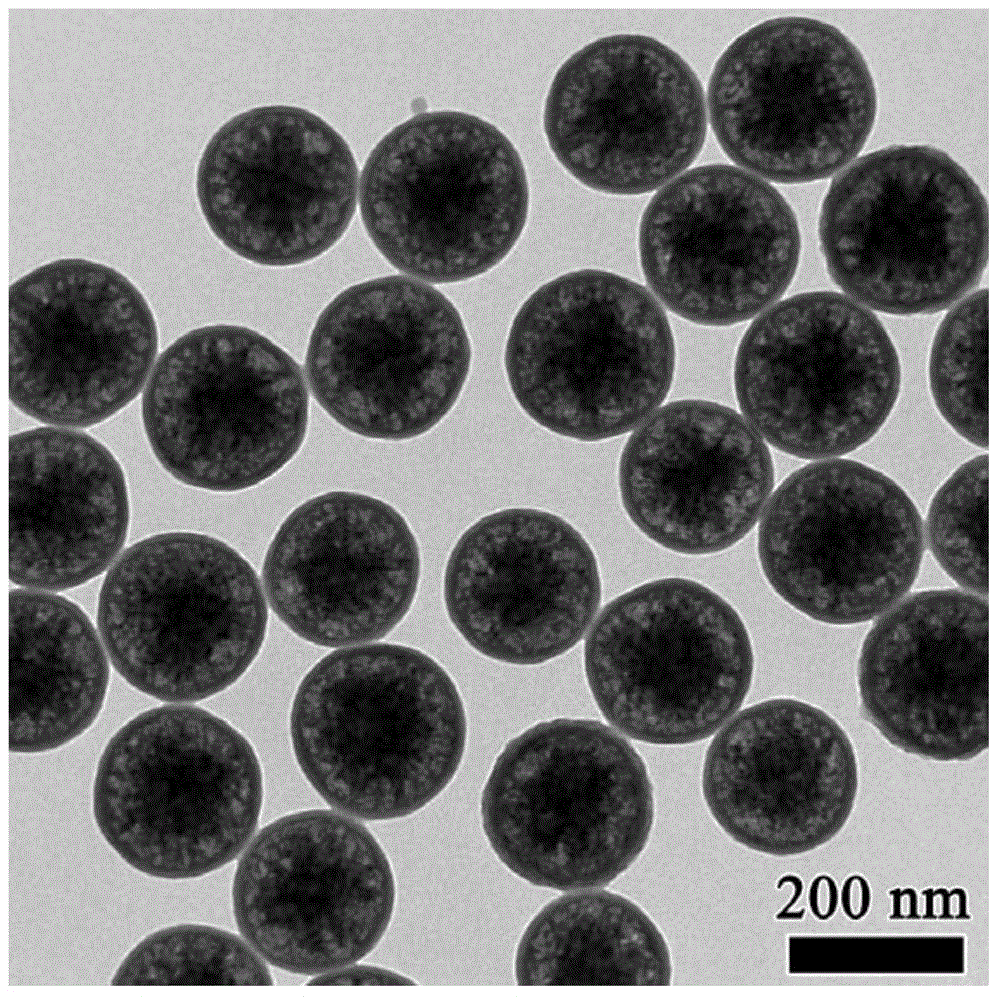
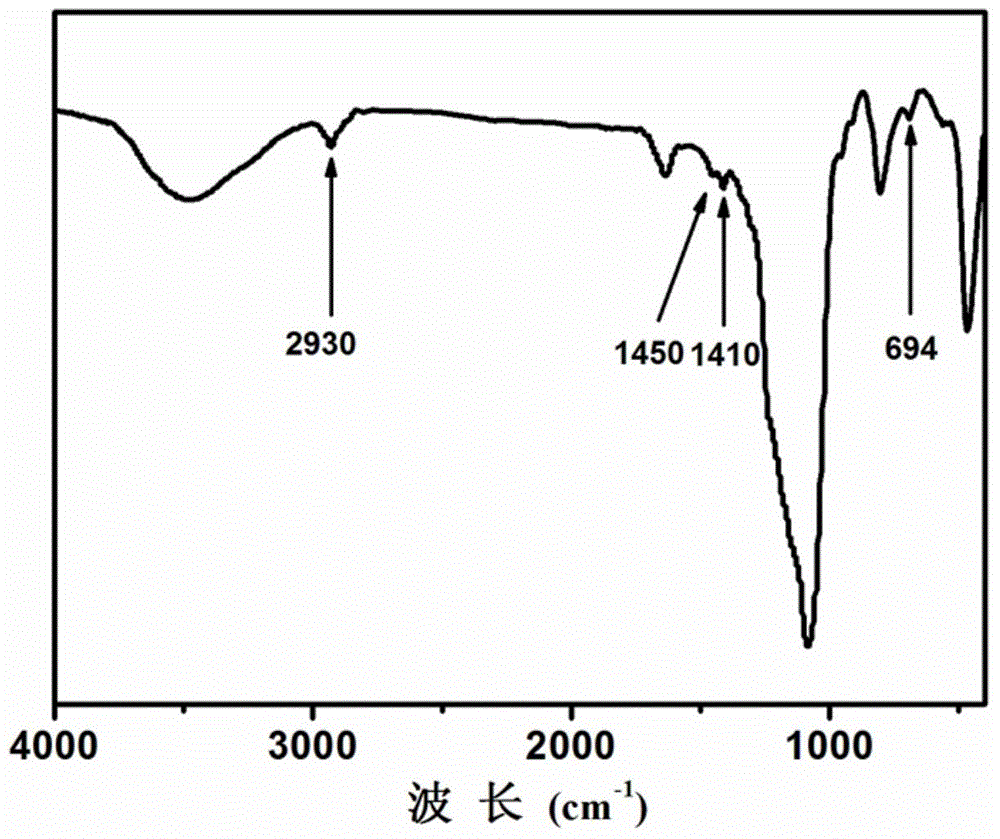
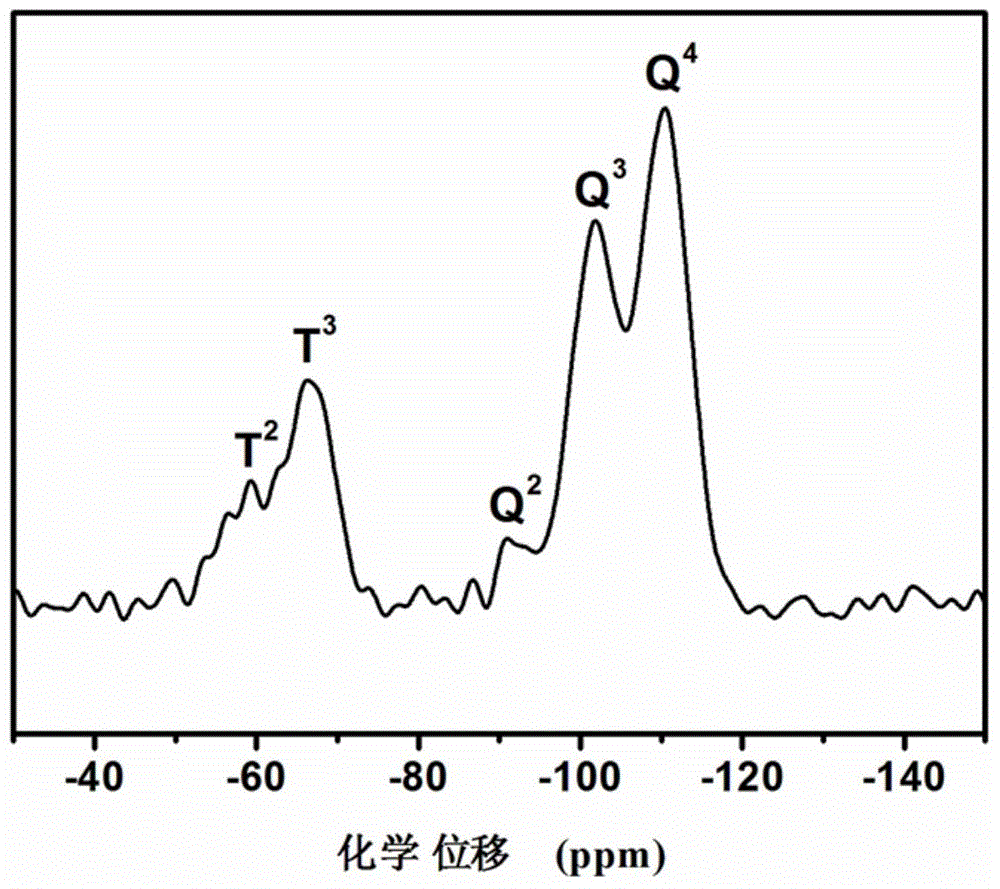
Long-chain-thioether-bond-containing mesoporous organic-inorganic hybrid ball of core-hollow-shell structure and preparation method thereof
A chain thioether and mesoporous technology, which is applied in the field of mesoporous organic-inorganic hybrid spheres and its preparation, can solve problems such as the inability to prepare silicon-based materials, and overcome the inability to transform into egg yolk-eggshell mechanisms and synthesis methods The effect of simplicity and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Mix 1ml of concentrated ammonia water (the NH contained in the concentrated ammonia water 3 The mass percent concentration is 25%) mixed with 30ml ethanol and 75ml water, then 0.16g hexadecyltrimethylammonium bromide was dissolved in the mixed solution, and 0.25ml orthosilicon was mixed at 35°C with 980rpm stirring Acid tetraethyl ester and 0.1ml of bis-[3-(triethoxysilyl)propyl]tetrasulfide were added to the aqueous alcohol solution and reacted for 24h to obtain a white solution. The solution was centrifuged and washed once with ethanol;
[0039] (2) Add ethanol to the product obtained in step (1) to dilute to 30ml, take 5ml and centrifuge, disperse in 30ml of water, transfer to a reaction kettle, and react overnight in an oven at 150°C and 1.0MPa;
[0040] (3) The reaction product obtained in step (2) was centrifuged, washed once with ethanol, transferred to a mixed solution of 500 μl concentrated hydrochloric acid and 250 ml ethanol, and heated in a water bath a...
Embodiment 2
[0046] (1) Mix 1ml of concentrated ammonia water (the NH contained in the concentrated ammonia water 3 The mass percent concentration is 25%) mixed with 30ml ethanol and 75ml water, then 0.08g hexadecyltrimethylammonium bromide was dissolved in the mixed solution, and 0.25ml orthosilicon Acid tetraethyl ester and 0.1ml of bis-[3-(triethoxysilyl)propyl]tetrasulfide were added to the aqueous alcohol solution and reacted for 24h to obtain a white solution. The solution was centrifuged and washed once with ethanol;
[0047] (2) Add ethanol to the product obtained in step (1) to dilute to 30ml, take 5ml and centrifuge, disperse in 30ml of water, transfer to a reaction kettle, and react overnight in an oven at 150°C and 1.0MPa;
[0048] (3) The reaction product obtained in step (2) was centrifuged, washed once with ethanol, transferred to a mixed solution of 500 μl concentrated hydrochloric acid and 250 ml ethanol, and heated in a water bath at 60° C. for 3 hours;
[0049] (4) Ste...
Embodiment 3
[0052] (1) Mix 1ml of concentrated ammonia water (the NH contained in the concentrated ammonia water 3 The mass percentage concentration is 25%) mixed with 30ml ethanol and 75ml water, then 0.12g hexadecyltrimethylammonium bromide was dissolved in the mixed solution, and 0.25ml orthosilicon Acid tetraethyl ester and 0.1ml of bis-[3-(triethoxysilyl)propyl]tetrasulfide were added to the aqueous alcohol solution and reacted for 24h to obtain a white solution. The solution was centrifuged and washed once with ethanol;
[0053] (2) Add ethanol to the product obtained in step (1) to dilute to 30ml, take 5ml and centrifuge, disperse in 30ml of water, transfer to a reaction kettle, and react overnight in an oven at 150°C and 1.0MPa;
[0054] (3) The reaction product obtained in step (2) was centrifuged, washed once with ethanol, transferred to a mixed solution of 500 μl concentrated hydrochloric acid and 250 ml ethanol, and heated in a water bath at 60° C. for 3 hours;
[0055] (4) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com