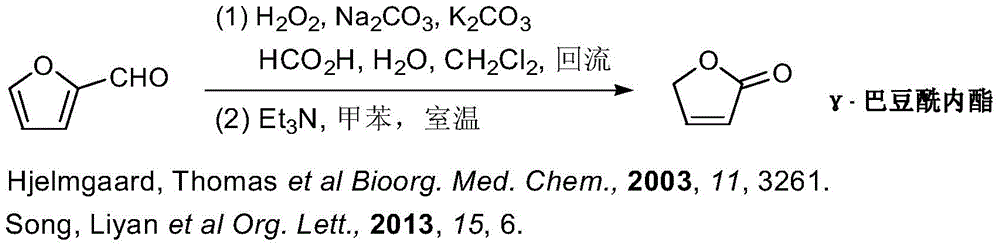
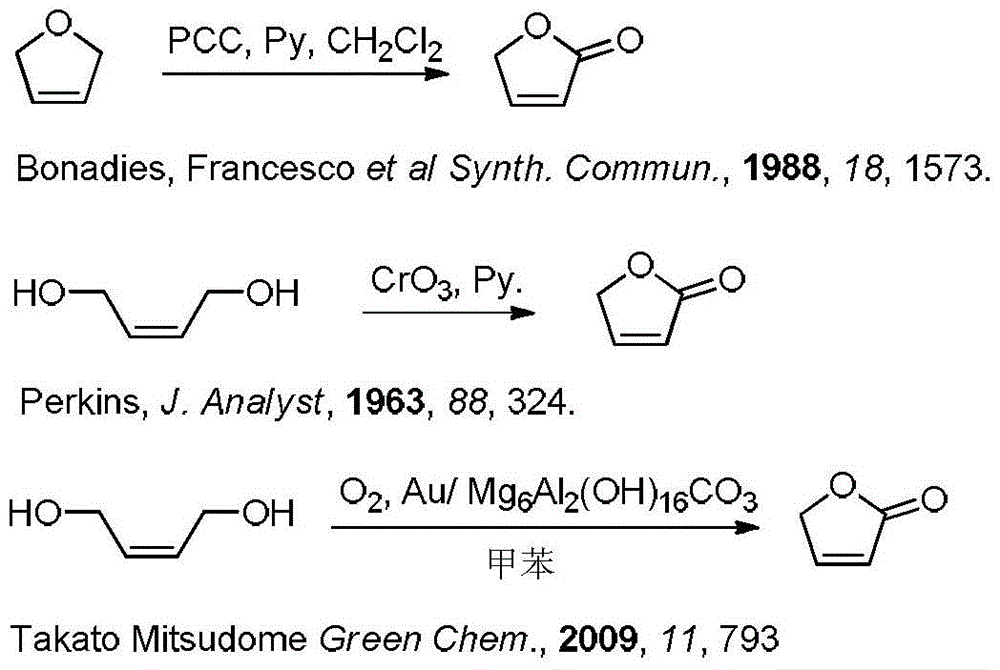
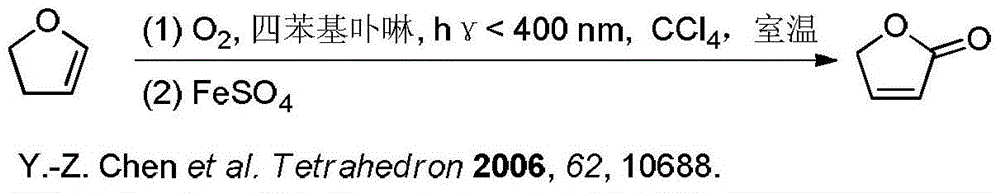A kind of synthetic method of γ-crotonyl lactone and its derivatives
A technology for the synthesis of crotonyl lactone and its method, which is applied in the field of synthesis of γ-crotonyl lactone and its α-derivatives, can solve the problems of difficult large-scale production and use, high price, etc., and achieve easy industrial production , cheap reagents, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] At room temperature, 1 (26 g, 0.2 mol) was added to a solution of acetone (1000 mL) to form a molar concentration of 0.2 mol.L -1 organic solution, then add H 2 O (10 mL), add hydrochloric acid (1 mL) drop by drop under vigorous stirring, after the addition is complete, continue stirring at room temperature for 12 hours, carefully add solid sodium bicarbonate to neutralize to neutral, filter with celite, wash with dichloromethane , combine the filtrate, remove most of the organic solvent by distillation under reduced pressure, add water, extract the water layer with dichloromethane, combine the organic layer, dry over anhydrous magnesium sulfate, filter, remove the solvent by distilling the filtrate under normal pressure, the remaining liquid column chromatography (dichloromethane Methane / methanol) afforded γ-crotonyl lactone 7 (14 g, 82%).
[0053]
[0054] 1 H NMR (300 MHz, CDCl 3 ): δ 4.91 (dd, 2H, J = 2.4, 1.6 Hz), 6.15 (dt, 1H, J = 6.0, 1.6 Hz), 7.57 (m, 1H)....
Embodiment 2
[0056] At room temperature, 1 (13 g, 0.1 mol) was added to a solution of acetone (100 mL) to form a molar concentration of 1 mol.L -1 organic solution, then add H 2 O (10 mL), add hydrochloric acid (1 mL) dropwise under vigorous stirring, after the addition is complete, continue to stir at room temperature for 8 hours, carefully add solid sodium bicarbonate to neutralize to neutral, filter with diatomaceous earth, and wash with dichloromethane , combine the filtrate, remove most of the organic solvent by distillation under reduced pressure, add water, extract the water layer with dichloromethane, combine the organic layer, dry over anhydrous magnesium sulfate, filter, remove the solvent by distilling the filtrate under normal pressure, the remaining liquid column chromatography (dichloromethane Methane / methanol) afforded γ-crotonyl lactone 7 (6.9 g, 82%).
[0057]
[0058] 1 H NMR (300 MHz, CDCl 3 ): δ 4.91 (dd, 2H, J = 2.4, 1.6 Hz), 6.15 (dt, 1H, J = 6.0, 1.6 Hz), 7.57 ...
Embodiment 3
[0060] At room temperature, 1 (13 g, 0.1 mol) was added into acetone (1000 mL) solution to form a molar concentration of 0.1 mol.L -1 organic solution, then add H 2 O (10 mL), add hydrochloric acid (1 mL) dropwise under vigorous stirring, after the addition is complete, continue stirring at room temperature for 16 hours, carefully add solid sodium bicarbonate to neutralize to neutral, filter with Celite, wash with dichloromethane , combine the filtrate, remove most of the organic solvent by distillation under reduced pressure, add water, extract the water layer with dichloromethane, combine the organic layer, dry over anhydrous magnesium sulfate, filter, remove the solvent by distilling the filtrate under normal pressure, the remaining liquid column chromatography (dichloromethane Methane / methanol) afforded γ-crotonolactone 7 (7.1 g, 84%).
[0061]
[0062] 1 H NMR (300 MHz, CDCl 3 ): δ 4.91 (dd, 2H, J = 2.4, 1.6 Hz), 6.15 (dt, 1H, J = 6.0, 1.6 Hz), 7.57 (m, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com