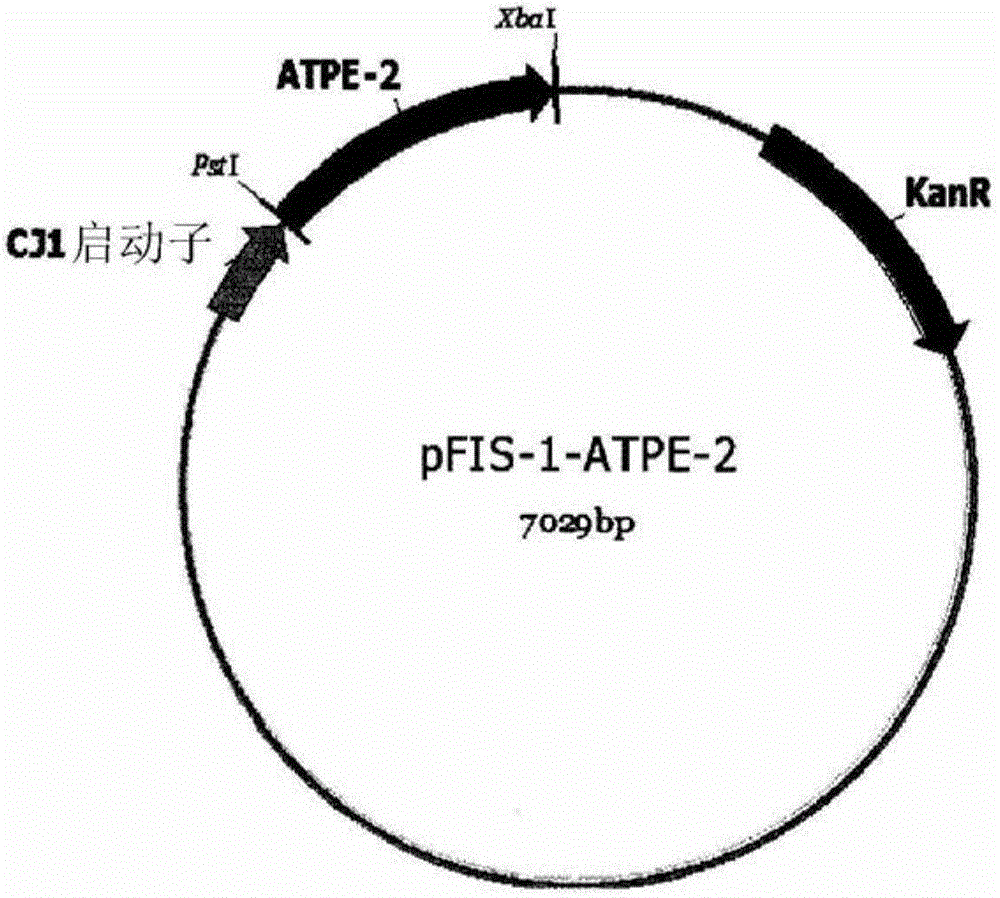
D-psicose 3-epimerase mutant with improved thermal stability, and continuous production of d-psicose using same
A technology of epimerase and psicose, which is applied in the direction of racemase/epimerase, isomerase, and the use of vectors to introduce foreign genetic material, can solve problems such as side effects, and achieve extended half-life, Effects of reducing production time and cost and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0056] Preparation of D-psicose 3-epimerase variants with improved thermostability by random mutagenesis
[0057] D-psicose 3-epimerase derived from Agrobacterium tumefaciens ATCC33970 (having an amino acid sequence identical to SEQ ID NO: 1 disclosed in Korean Patent Publication No. 10-2011-0035805A) was used A library of D-psicose 3-epimerase variants was constructed by error-prone polymerase chain reaction (error-prone PCR) as a template.
[0058] Specifically, a typical PCR mutagenesis kit that results in two to three mutations per 1000 base pairs is employed. As primers, oligonucleotides introduced into the restriction enzyme recognition site sequences of NcoI and PstI restriction enzymes were used to perform polymerase chain reaction to construct genes encoding D-psicose 3-epimerase variants library, the variants were then inserted into E. coli BL21.
[0059] LB medium containing 50 µg / ml ampicillin was used to culture Escherichia coli BL21 containing a plasmid contain...
example 2
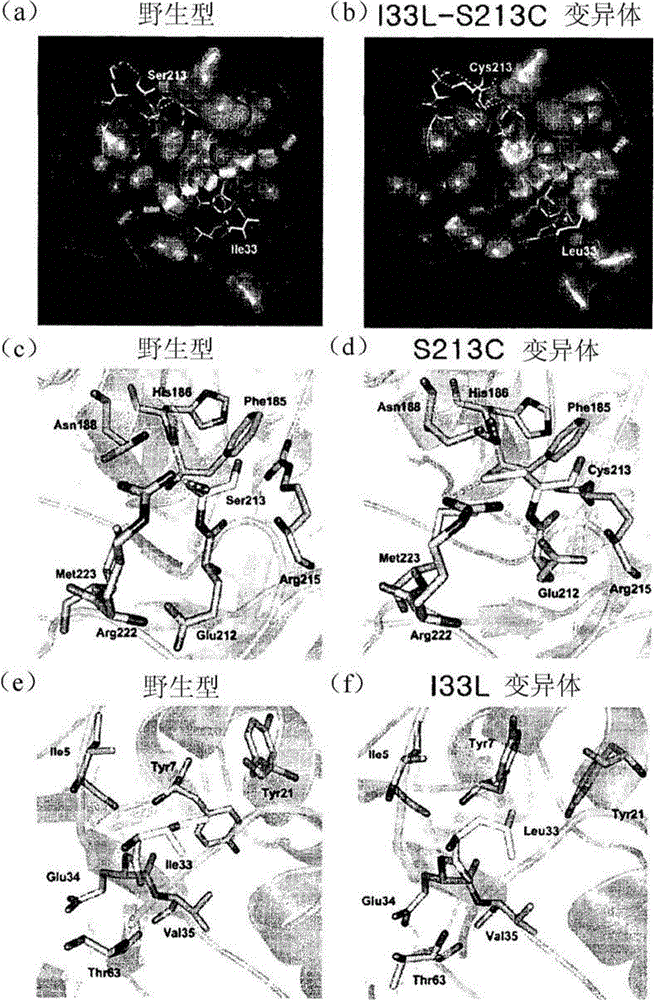
[0068] Preparation of D-psicose 3-epimerase variants with improved thermostability using rational design
[0069] In a similar manner to Example 1, variants S213C and I33L were selected as variants with improved thermostability without significantly decreased activity. Based on the selected variants, amino acids at positions 33 and 213 of the amino acid sequence were subjected to site-directed mutagenesis.
[0070] Specifically, the polar and uncharged serine (Ser) at position 213 is replaced by polar and uncharged threonine (Thr), cysteine (Cys) or methionine (Met), respectively; The nonpolar amino acid proline (Pro); glutamic acid (Glu), which has a negative charge; and lysine (Lys), which has a positive charge, are substituted.
[0071] The non-polar isoleucine at position 33 is replaced by polar and uncharged cysteine; non-polar valine (Val), leucine or proline; negatively charged valley amino acid; and a positively charged lysine substitution.
[0072] The relative a...
comparative experiment example 1
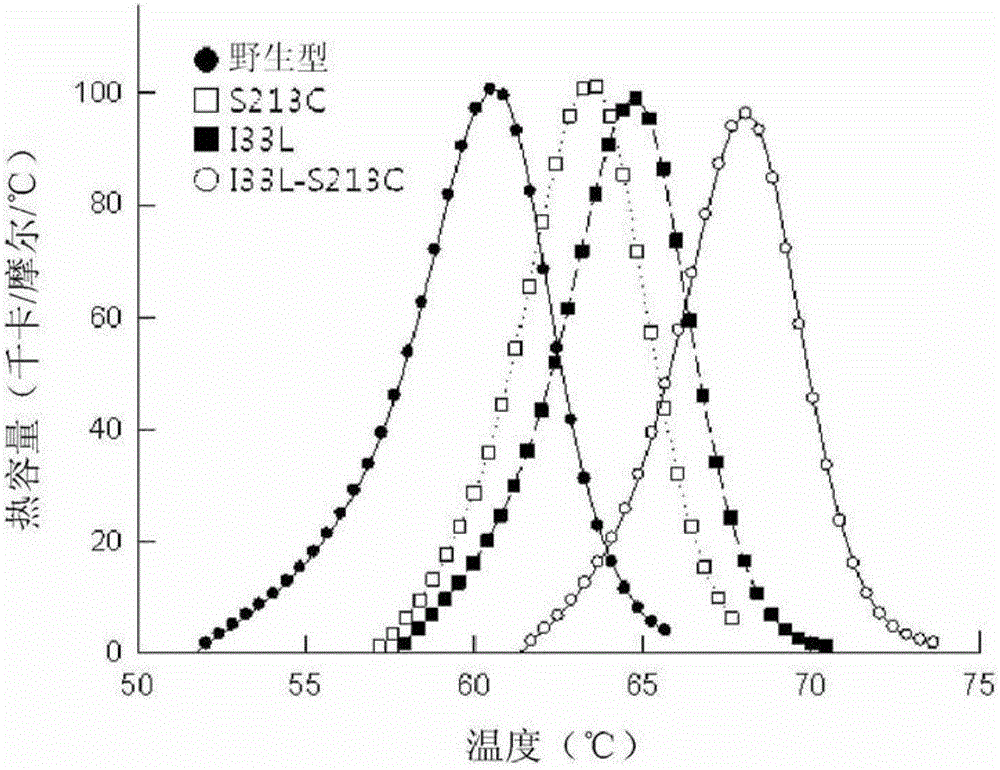
[0079] Comparison of the apparent melting temperatures of wild-type D-psicose 3-epimerase and D-psicose 3-epimerase variants according to Example 2
[0080] To determine the thermostability of wild-type D-psicose 3-epimerase and D-psicose 3-epimerase variants according to Example 2 (i.e. I33L, S213C and I33L-S213C) To measure the apparent melting temperature (T m ).
[0081] Therefore, it can be seen that the apparent melting temperatures arranged in ascending order are wild type figure 2 ). Specifically, the apparent melting temperature was increased by about 4.3°C in the case of the S213 variant and by about 7.6°C in the case of the I33L-S213C variant compared to the wild type.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com