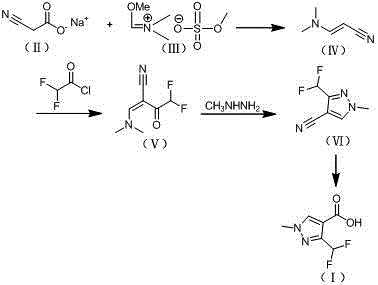
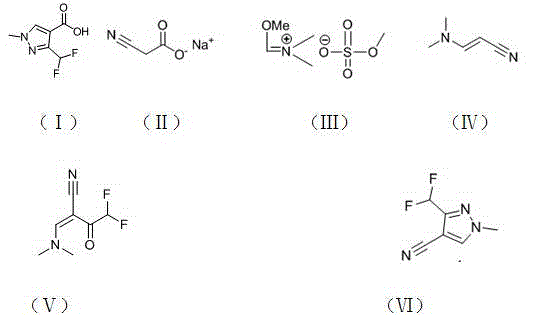
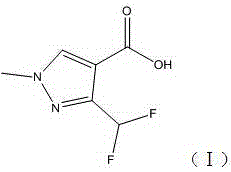
Preparation method of 3-difluoromethyl-1-methyl-1H-pyrazolyl-4-carboxylic acid
A technology of difluoromethyl and dimethylaminoacrylonitrile, which is applied in the field of synthesis of fine chemical intermediates, can solve the problems of high price of ethyl difluoroacetoacetate, unsuitability for industrial production, and high cost of industrial production, and achieve cost Low cost, low equipment requirements, and short process routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of 3-difluoromethyl-1-methyl-1H-pyrazole-4-carboxylic acid (I)
[0034] 1) Preparation of N,N-dimethylaminoacrylonitrile (IV): Put 9.8g (92mmol) of sodium cyanoacetate and 50mL of toluene into a 100mL four-necked flask, and control the temperature at 40°C Add 55g (228mmol) of the imide salt shown in the formula (Ⅲ) dropwise, about 3 hours after the dropwise addition, keep the temperature for 6 hours, add 60mL of 5% sodium hydroxide aqueous solution to the feed solution, and stir for 5 minutes , layered extraction, the obtained toluene layer was dried with anhydrous sodium sulfate, concentrated to obtain 9.2g of N,N-dimethylaminoacrylonitrile shown in formula (IV), and the content of the product detected by GC (gas chromatography) was 94.2 %, the yield is 98.3%. 1 H-NMR (CDCl 3 , 500 MHz): δ=2.87 (6H, s, CH3), 3.60 (1H, d, CH), 6.91 (1H, d, CH);
[0035] In above-mentioned embodiment, replace toluene with any one in benzene, dichloromethane, she...
Embodiment 2
[0043] Example 2: Preparation of 3-difluoromethyl-1-methyl-1H-pyrazole-4-carboxylic acid (I)
[0044] 1) Preparation of N,N-dimethylaminoacrylonitrile (Ⅳ): Put 4.9g (46mmol) sodium cyanoacetate and 40mL ethanol into a 100mL four-neck flask, and add 27g (113mmol) dropwise at a temperature of about 30°C, such as The imine salt shown in formula (Ⅲ) was added dropwise in about 2 hours, and the reaction was incubated for 8 hours. 50 ml of 5% aqueous sodium hydroxide solution was added to the feed liquid, stirred for 5 minutes, extracted with toluene, and the obtained toluene The layer was dried over anhydrous sodium sulfate and concentrated to obtain 4.1 g of N,N-dimethylaminoacrylonitrile represented by formula (IV). The product content was 95.1% and the yield was 97.4% as detected by GC (gas chromatography). 1 H-NMR (CDCl 3 , 500 MHz): δ=2.85 (6H, s, CH3), 3.61 (1H, d, CH), 6.93 (1H, d, CH);
[0045] 2) Preparation of 2-((dimethylamino)methylene)-4,4-difluoro-3-carbonylbutyroni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com