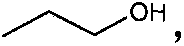
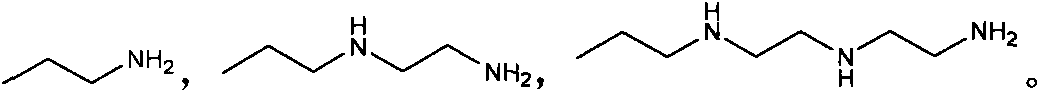Mercaptobenzothiazolyl imidazoline derivative, and preparation method and application thereof
A technology of mercaptobenzothiazole and derivatives, applied in lubricating compositions, petroleum industry, organic chemistry, etc., can solve problems such as restricted use, poor solubility, etc., and achieve good extreme pressure, high yield, and easy control of reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0020] Example 1: 40g of 2-mercaptobenzothiazole and 11.0g of sodium hydroxide aqueous solution were mixed and heated for 3-4h (above 90°C), and 25g of chloroacetic acid aqueous solution was slowly added dropwise (30min) for 3-4h. Product purification: Cool, add HCL dropwise until the pH is 2-3 (a large amount of white precipitate), filter and wash with hot water above 80°C for 3 times, dry, and the measured melting point is 152°C. Then add xylene as solvent, water carrier and diethylenetriamine to a 250ml three-necked flask, react at 140-160°C for 4 hours to remove the water generated in the reaction, and react at 190-210°C for 2 hours to remove the water generated in the reaction Distill water under reduced pressure to obtain a light yellow-brown viscous liquid 2-Mercaptobenzothiazoleacetic acid imidazole ethylamine.
example 2
[0021] Example 2: 40g of 2-mercaptobenzothiazole and 11g of sodium hydroxide aqueous solution were mixed and heated for 3-4h (above 90°C), and 25g of chloroacetic acid aqueous solution was slowly added dropwise (30min) for 3-4h. Product purification: Cool, add HCL dropwise until the pH is 2-3 (a large amount of white precipitate), filter and wash with hot water above 80°C for 3 times, dry, and the measured melting point is 152°C. Then add xylene as solvent and water-carrying agent, N-hydroxyethylethylenediamine to a 250ml three-necked flask, react at 140-160°C for 4h to remove the water generated in the reaction, and react at 190-210°C for 2h to remove The water produced in the reaction was distilled under reduced pressure to obtain light yellow-brown viscous liquid 2-mercaptobenzothiazoleacetic acid imidazolyl ethanol.
[0022] All target compounds were determined by Spectrum One infrared spectrometer to have imidazole rings in the synthesized substances, and the C, H, and N ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com