Biodegradable material and method for producing biodegradable material
A biodegradable compound technology, applied in tissue regeneration, bandages, surgery, etc., can solve problems such as deformation of polymer particles, and achieve the effects of improving softness, shear strength, and shape recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0217] 10.0 g of branched chain polymer a1, 8-branched PEG (SUNBRIGHT (registered trademark) HGEO5000; manufactured by NOF Corporation) and hydroxycarboxylic acid a2, namely 22.0 g of lactide (PURASORB L; manufactured by PURAC BIOMATERIALS) were extracted. into an eggplant-shaped bottle. These were melt-mixed at 120° C. under an argon atmosphere, and 0.94 mL of tin (II) octoate toluene solution was added as a catalyst (tin (II) octoate (manufactured by Sigma-Aldrich Co.,) was dissolved in toluene (Wako Pure Chemical Industries, Ltd. Kogyo Co., Ltd., adjusted to a concentration of 0.16 g / mL), and a copolymerization reaction was performed under normal pressure for 20 hours to obtain an unpurified multi-component compound A1.
[0218] The obtained unrefined multi-component compound A1 was added dropwise to 100 mL of diethyl ether, and after the precipitate and liquid components separated from diethyl ether were collected, they were further washed with 50 mL of diethyl ether for 3...
Embodiment 2)
[0227] Respectively change the consumption of solution 1 to 0.570mL, change the consumption of solution 2 to 0.430mL, change the consumption of DMAP solution to 0.022mL, change the consumption of EDC to 0.038mL, except that, carry out the same as Example 1 Operation, thereby obtaining biodegradable membrane 2 and acetonitrile-containing membrane 2.
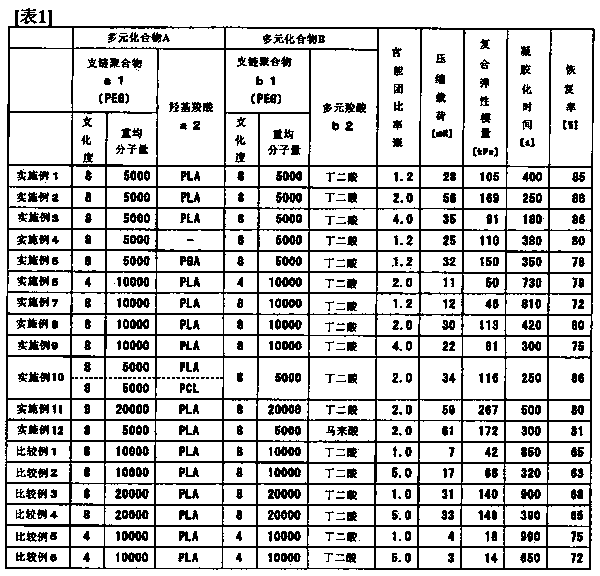
[0228] These biodegradable film 2 and acetonitrile-containing film 2 were evaluated in the same manner as in Example 1, and the results are shown in Table 1.
[0229] As shown in Table 1, the biodegradable film 2 has a large compressive load and a high recovery rate. In addition, the complex elastic modulus of the acetonitrile-containing membrane 2 is large, and the gelation time is also fast.
Embodiment 3)
[0231] Change the consumption of solution 1 to 0.399mL, change the consumption of solution 2 to 0.601mL, change the consumption of DMAP solution to 0.030mL, and change the consumption of EDC to 0.053mL, except that, carry out the same as Example 1 Operation, thereby obtaining biodegradable membrane 3 and acetonitrile-containing membrane 3.
[0232] These biodegradable films 3 and acetonitrile-containing films 3 were evaluated in the same manner as in Example 1, and the results are shown in Table 1.
[0233] As shown in Table 1, the biodegradable film 3 has a large compressive load and a high recovery rate. In addition, the complex elastic modulus of the acetonitrile-containing membrane 3 is large, and the gelation time is also fast.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com