EGFR-oriented monoclonal antibody modified PAMAM self-assembled transgenic composition and applications thereof
A composition and transgenic technology, applied in gene therapy, drug combination, medical preparations of non-active ingredients, etc., can solve the problems of increasing the steric hindrance of the combination of PAMAM molecules and DNA molecules, reducing transfection efficiency, and occurrence of hemolysis , to reduce cytotoxicity and hemolysis, promote tumor cell apoptosis, and inhibit tumor cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1, preparation mAb / PAMAM G5 / DNA complex
[0053] 1. PAMAM G5 / DNA complex
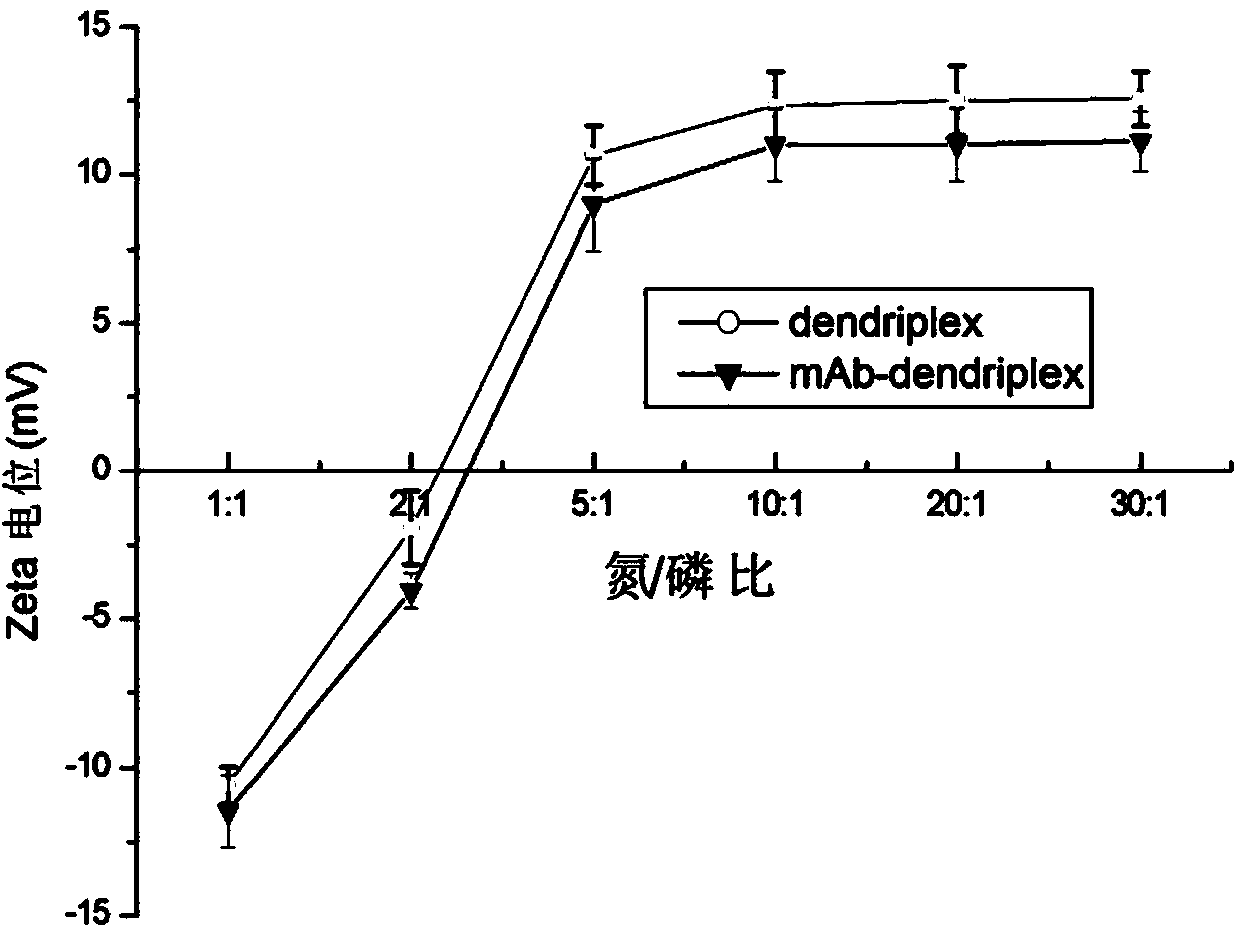
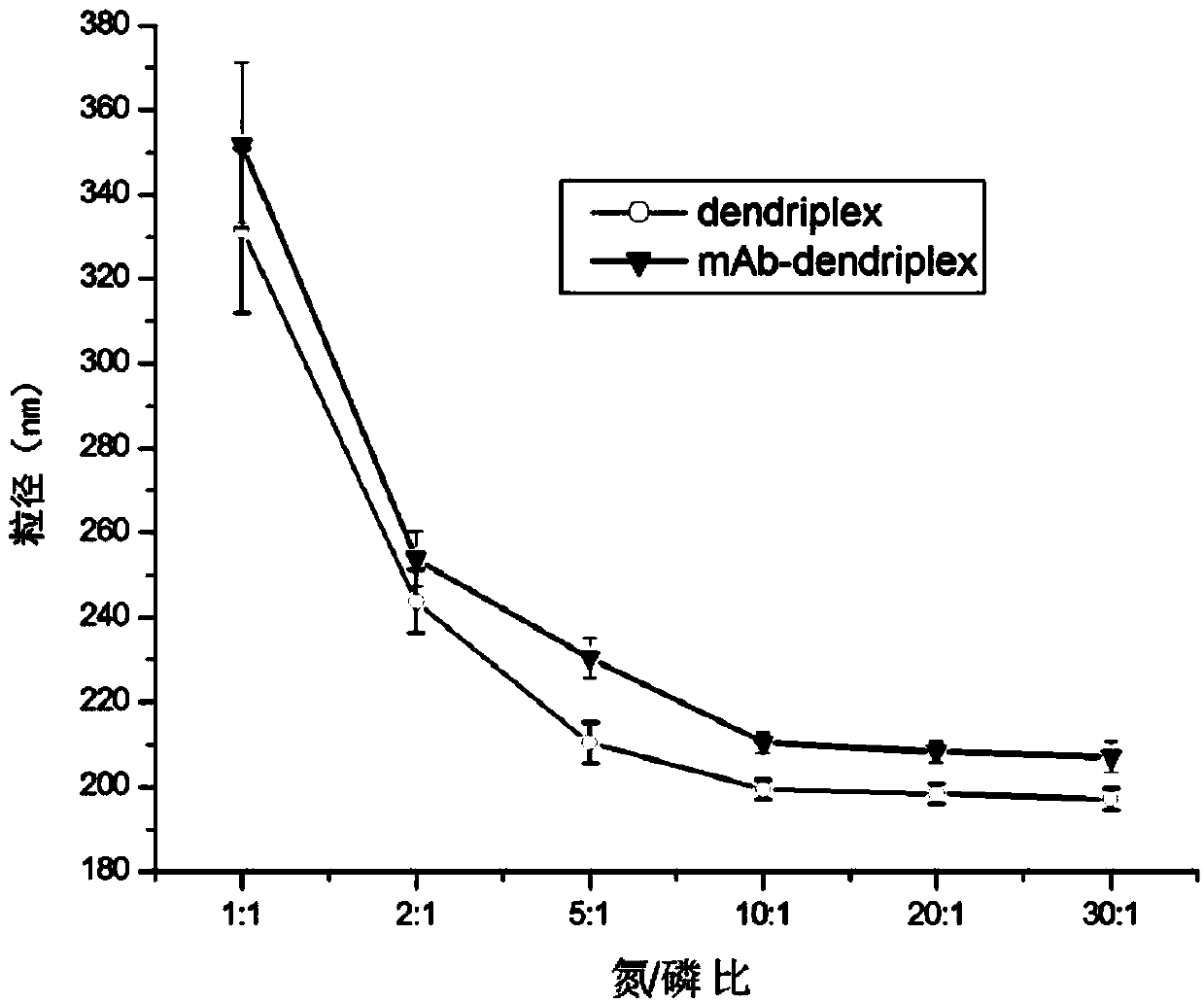
[0054] First, the 10 mg / mL stock solution of PAMAM G5 (number average molecular weight: 28824.81) prepared above was dissolved in sterilized distilled water, oscillated and mixed to prepare a 1 mg / mL working solution, and stored at 4°C for later use. Before transfection, take 1.0 μg of pEGFP-N1 plasmid DNA (pEGFP-N1 plasmid was purchased from Clontech Company) and put it into EP tubes, add 0.7 μL, 1.4 μL, 3.5 μL, 7 μL, 14 μL, 21 μL, 28 μL and 42 μL respectively at a concentration of 1 mg / Add 500 μL of opti-MEM medium (purchased from invitrogen, catalog number 11058-021) to 5 mL of PAMAM G5 working solution, mix well and incubate at room temperature for 30 min to form a PAMAM G5 / DNA complex. In the formed PAMAM G5 / DNA complex, the nitrogen / phosphorus ratios of PAMAM G5 and DNA were 1:1, 2:1, 5:1, 10:1, 20:1, 30:1, 40:1 and 60, respectively. :1.
[0055] 2. mAb / PAMAM G5 / DNA complex a...
Embodiment 2
[0070] Example 2, Cytotoxicity Evaluation of mAb / PAMAM G5 / DNA Complex
[0071] The mAb / PAMAM G5 / DNA complex prepared in the above example 1 was subjected to cytotoxicity experiments. In the mAb / PAMAM G5 / DNA complex, the nitrogen / phosphorus ratios of PAMAM and DNA were 1:1, 5:1, and 10:1, respectively. , 20:1, 40:1 and 60:1; the mass ratio of mAb to DNA is 1:1. The cell proliferation was detected by MTT assay, the specific method is as follows:
[0072] HepG2, MCF-7, and 293T cells (purchased from the Cell Center of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences) were inoculated into 96-well plates at 10,000 cells per well, and transfected according to the optimal mass ratio after 24 hours, and the cells were randomly divided into Normal control group, PAMAM G5 / DNA group, and mAb / PAMAM G5 / DNA transfection group (the plasmid concentration is 0.5 μg / mL, each group has 6 replicate wells, repeated 3 times, and treated for 24 hours). The morphological...
Embodiment 3
[0077] Example 3. Application of mAb / PAMAM G5 / DNA complex in targeting DNA into cells
[0078] 1. mAb / PAMAM G5 / DNA complex improves DNA transfection efficiency and cell targeting
[0079] 1. The mAb / PAMAM G5 / DNA complex introduced DNA into HepG2 cells expressing EGFR
[0080] Prepare following compound according to the method for embodiment 1:
[0081] mAb / PAMAM G5 / DNA complex: the nitrogen / phosphorus ratio of PAMAM to DNA is 10:1, and the mass ratio of mAb to DNA is 0 (without monoclonal antibody), 0.1:1, 0.5:1, 1:1, 2:1, 5:1;
[0082] mAb / PAMAM G5 / DNA complex: the nitrogen / phosphorus ratio of PAMAM to DNA is 20:1, and the mass ratio of mAb to DNA is 0 (without monoclonal antibody), 0.1:1, 0.5:1, 1:1, 2:1, 5:1;
[0083] mAb / PAMAM G5 / DNA complex: the nitrogen / phosphorus ratio of PAMAM to DNA is 40:1, and the mass ratio of mAb to DNA is 0 (without monoclonal antibody), 0.1:1, 0.5:1, 1:1, 2:1, 5:1;
[0084] The mAb / PAMAM G5 / DNA complex prepared in Example 1 and the above-m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com