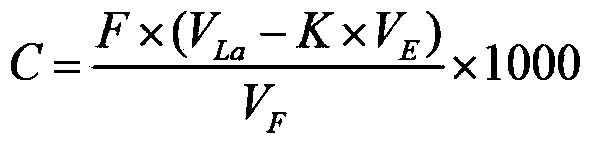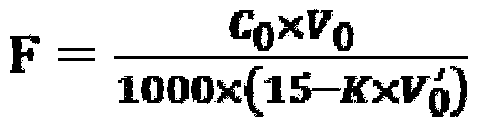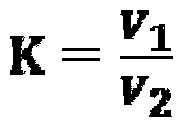Method for testing fluorine content in solution
A fluorine content and solution technology, which is applied in material analysis by observing the influence of chemical indicators, and analysis by chemical reaction of materials, etc., can solve problems such as inaccurate measurement, and achieve accurate and reproducible measurement results. Sexual and environmental pollution-free effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Take a 500mL Erlenmeyer flask, first add about 100mL of deionized water, then accurately pipette 1.0mL HF solution (known concentration is 45.0g / L) into the Erlenmeyer flask, shake well, add 2g of potassium nitrate, pipette accurately Put 75.00mL of lanthanum nitrate standard solution in a Erlenmeyer flask, adjust the pH value to 2.5, heat and boil for 2min, cool to room temperature, add 10mL of acetic acid-sodium acetate buffer solution, 5 drops of xylenol orange indicator, drop into the solution with EDTA standard solution From purple to bright yellow. Titrate in parallel three times, the amount of EDTA consumed is 61.9, 61.8., 62.0mL respectively, calculated to get F - The ion content is 45.4g / L.
Embodiment 2
[0067] Take a 500mL Erlenmeyer flask, first add about 100mL of deionized water, then accurately pipette 0.5mL of titanium milling solution containing HF concentration of about 47.0g / L, place it in the Erlenmeyer flask, shake well, add 2g of potassium nitrate, accurately Add 40.00mL of lanthanum nitrate standard solution to the Erlenmeyer flask, adjust the pH value to about 2.0 with 20g / L sodium hydroxide solution and 20% hydrochloric acid, add to boil for 2min, remove the water and cool to room temperature, add 10mL of acetic acid-sodium acetate buffer solution , 5 drops of xylenol orange indicator, drip with EDTA standard solution until the solution changes from purple red to bright yellow, which is the end point. The consumption of EDTA is 33.2, 33.5, 33.3mL respectively, and the F in the titanium milling solution is calculated. - The ion content is 47.4g / L.
Embodiment 3
[0069] Take a 500mL Erlenmeyer flask, first add about 100mL of deionized water, then accurately pipette 0.5mL of HF titanium milling solution with a concentration of 21.3g / L, place it in the Erlenmeyer flask, shake it well, add 2g of potassium nitrate, and accurately add 75.00 Put lanthanum nitrate standard solution in an Erlenmeyer flask, adjust the pH to about 2.0, heat and boil for 2 minutes, remove the water and cool to room temperature, add 10 mL of acetic acid-sodium acetate buffer solution, 5 drops of xylenol orange indicator, and drop to The solution turned from purple to bright yellow. Titrated in parallel three times, the amount of EDTA consumed was 84.8, 84.7, 84.5mL respectively, and the F in the titanium milling solution was calculated - The ion content is 21.0g / L.
[0070] Above embodiment does not limit the present invention, in actual operation, when preparing fluorine standard solution, lanthanum nitrate standard solution and EDTA standard solution at every t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com