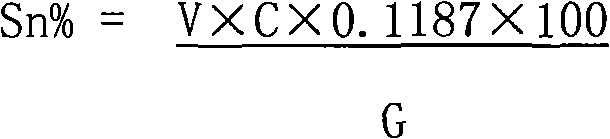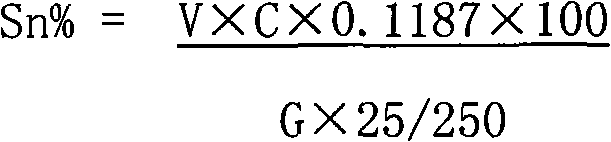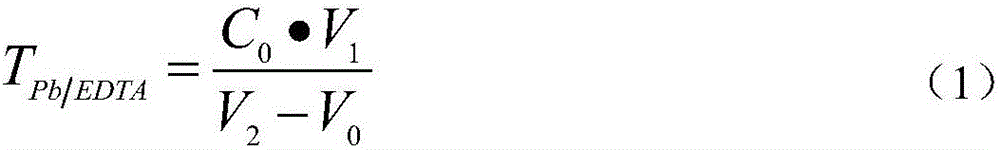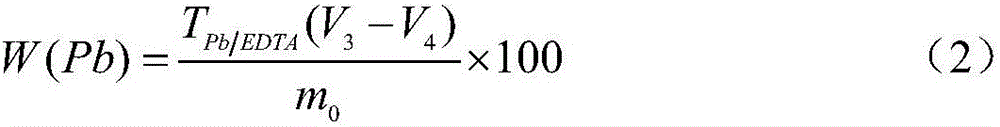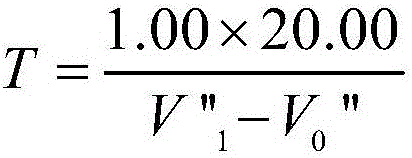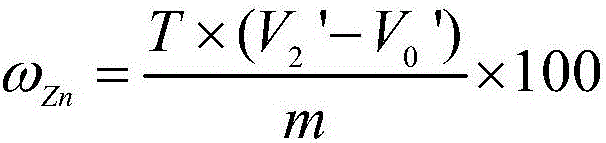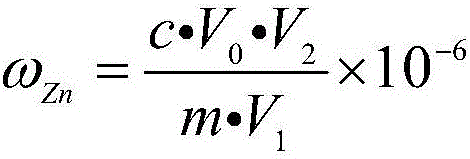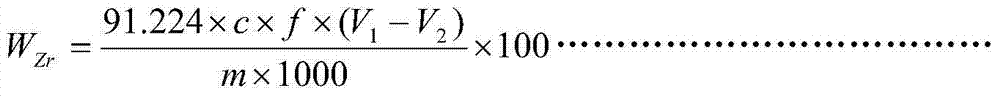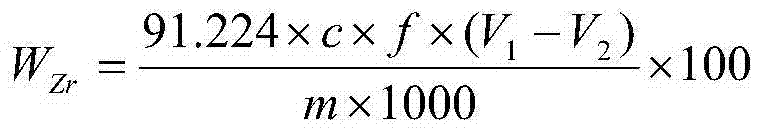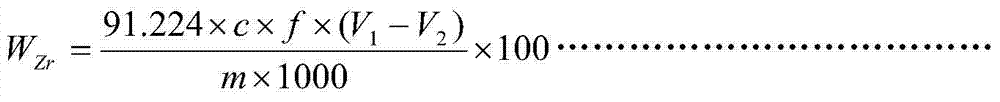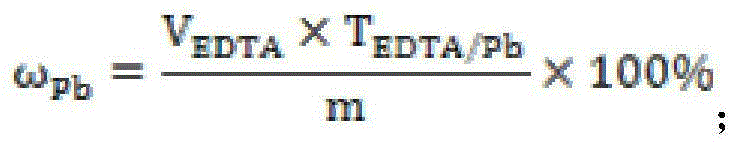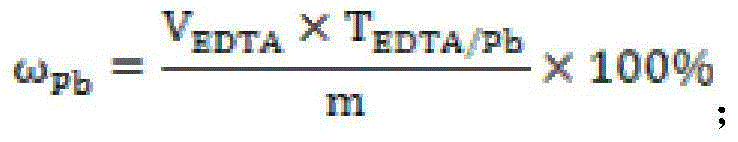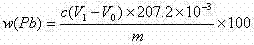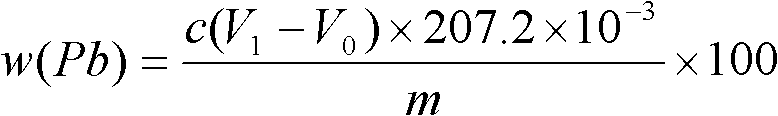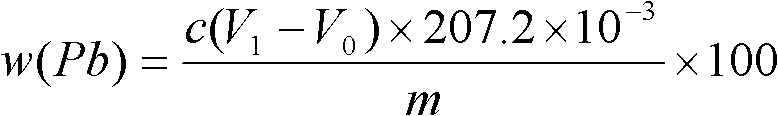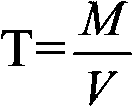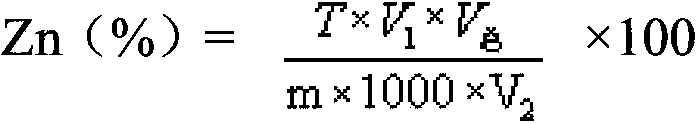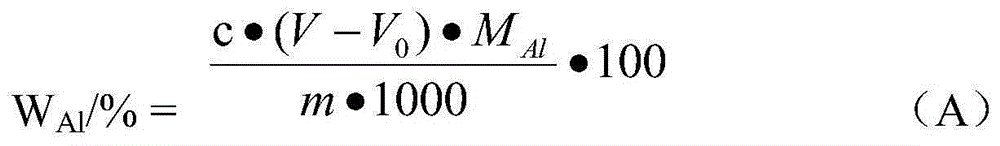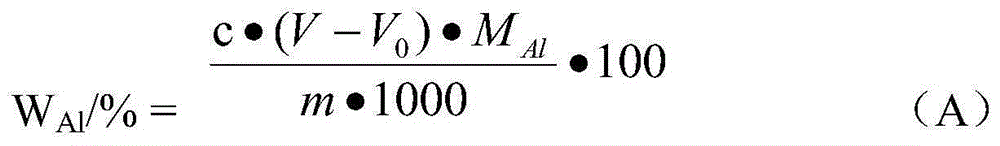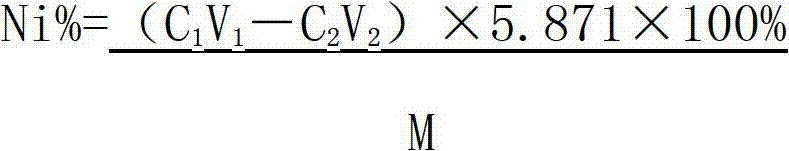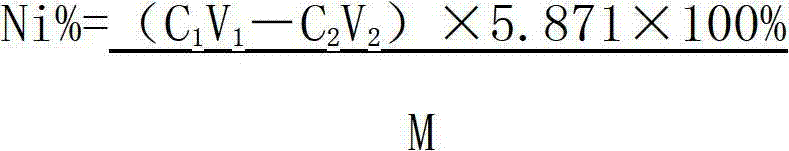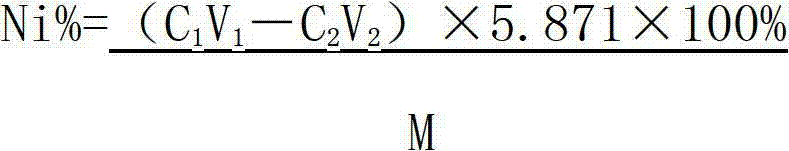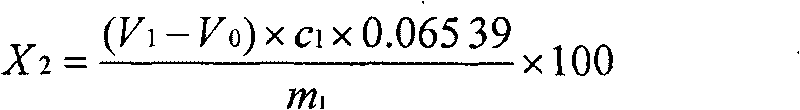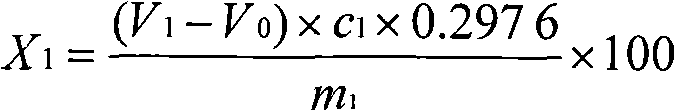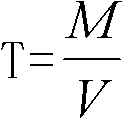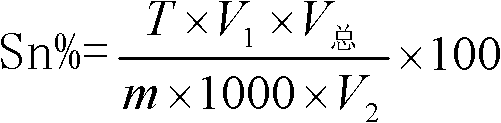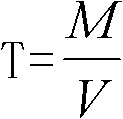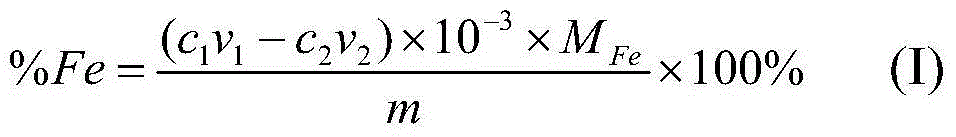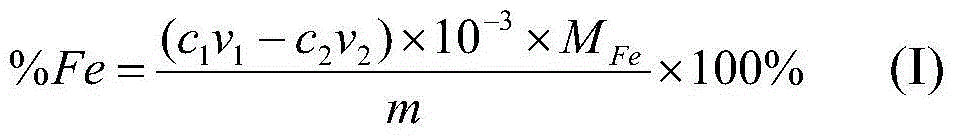Patents
Literature
111 results about "Xylenol orange" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Xylenol orange is an organic reagent, most commonly used as a tetrasodium salt as an indicator for metal titrations. When used for metal titrations, it will appear red in the titrand and become yellow once it reaches its endpoint. Historically, commercial preparations of it have been notoriously impure, sometimes consisting of as little as 20% xylenol orange, and containing large amounts of semi-xylenol orange and iminodiacetic acid. Purities as high as 90% are now available.
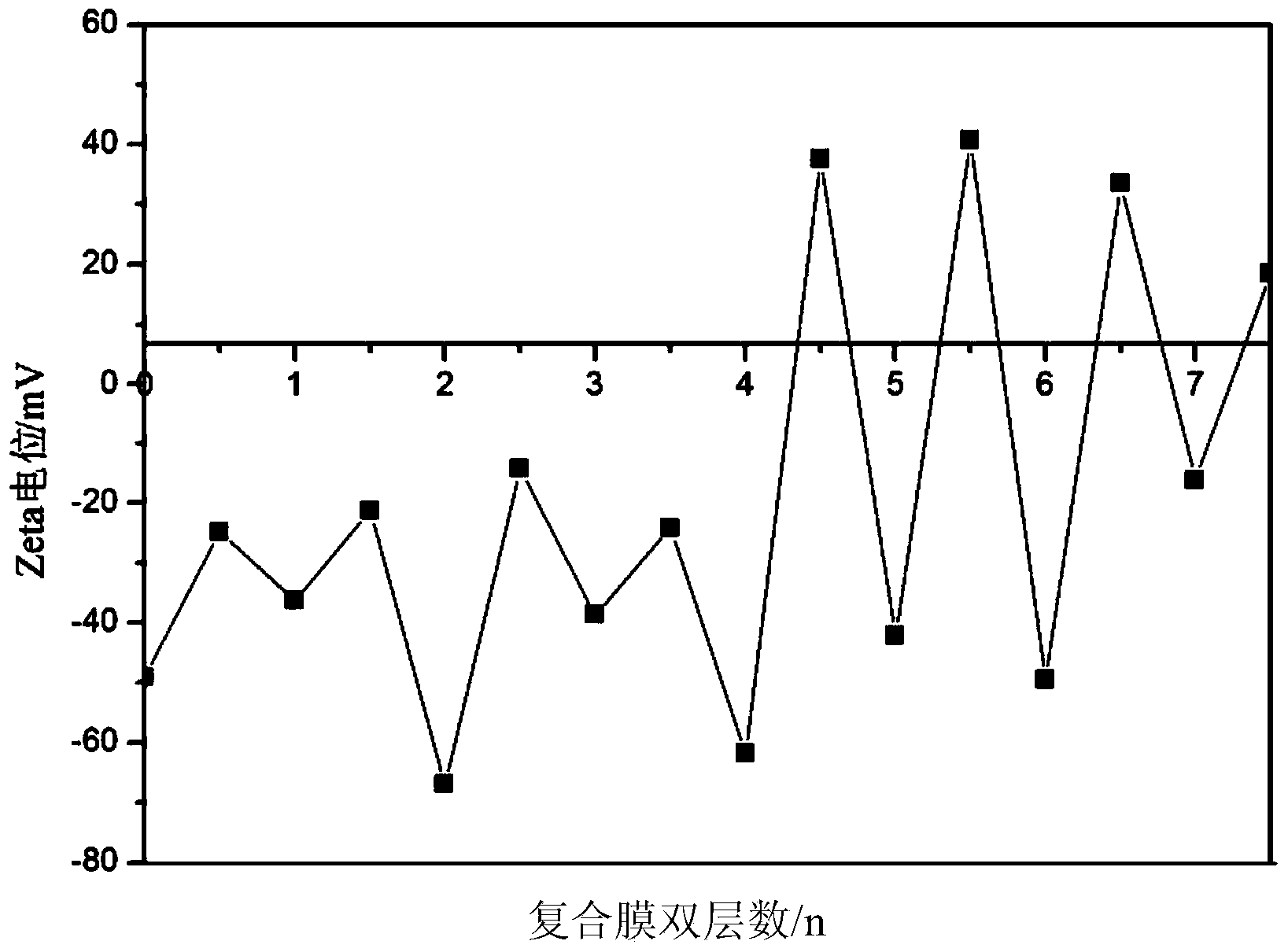
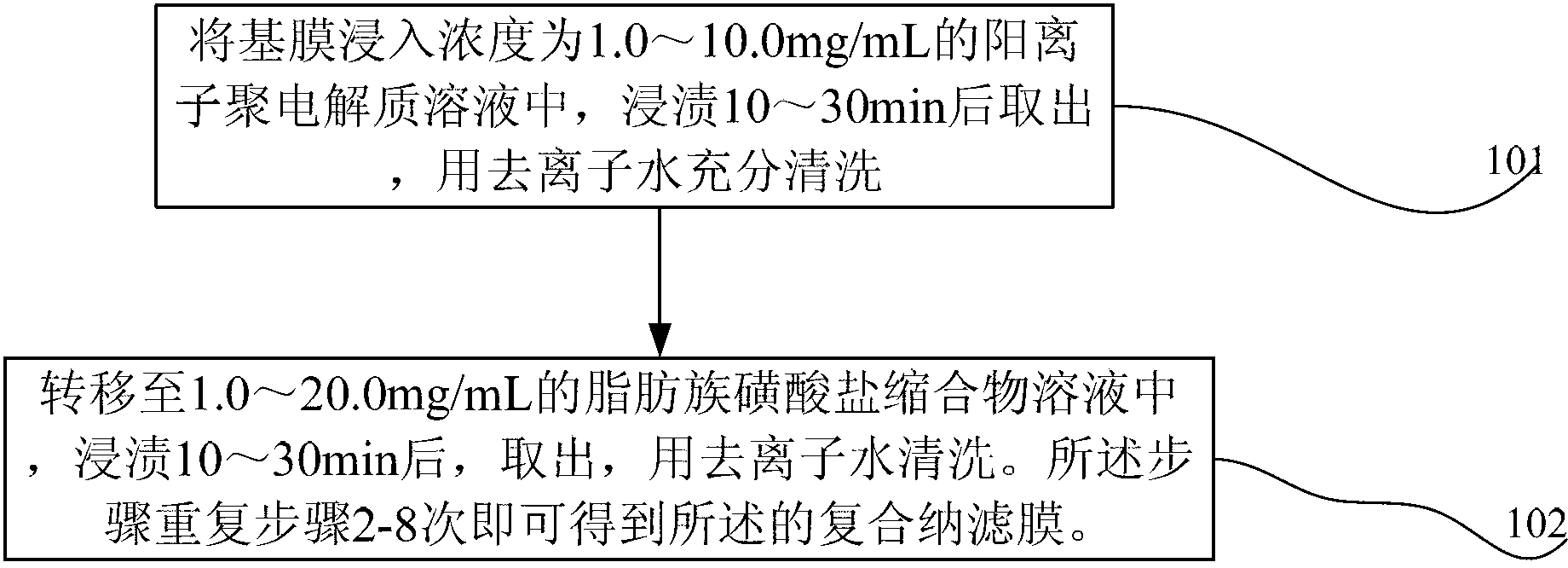
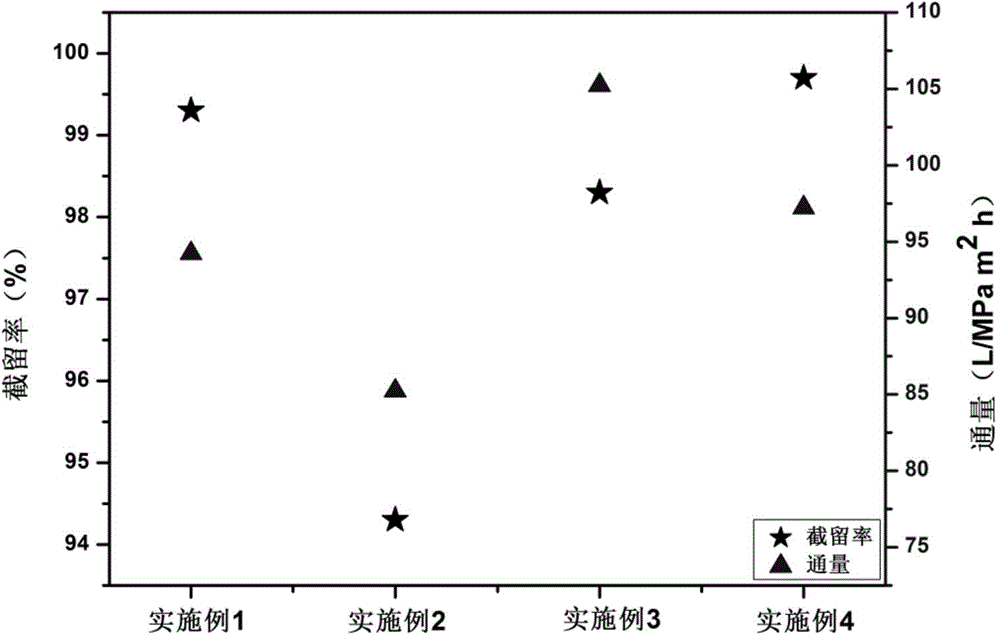
Layer by layer self-assembly compound nanofiltration membrane based on natural cellulose polyelectrolyte and preparation method
ActiveCN103551049AStrong anti-pollutionImprove interception effectSemi-permeable membranesCelluloseUltrafiltration
The invention discloses a layer by layer self-assembly compound nanofiltration membrane based on natural cellulose polyelectrolyte and a preparation method, belonging to the technical field of membrane separation. The preparation method comprises the following steps: firstly, adopting an ultrafiltration membrane as a base membrane for preparing a compound nanofiltration membrane, carrying out chemical modification on the surface of the base membrane to ensure that the surface of the base membrane has a charge property so as to generate static reaction with a polyelectrolyte; and secondly, through alternating depositing anionic and cationic polyelectrolytes, preparing the compound nanofiltration membrane through a layer by layer self-assembly method. According to the preparation method, the adopted cationic polyelectrolyte is a natural cellulose polyelectrolyte; compared with a currently used synthetic polyelectrolyte, the natural cellulose polyelectrolyte is lower in cost, and is an environmental-friendly resource; and the prepared compound nanofiltration membrane containing natural cellulose is good in hydrophilcity and charge performance so that the surface of the membrane is good in pollution resistance; and the compound nanofiltration membrane has a good retaining property for dye molecules such as divalent metal ions such as Ni<2+>, xylenol orange sodium salt, and rhodamine B.
Owner:BEIJING UNIV OF TECH
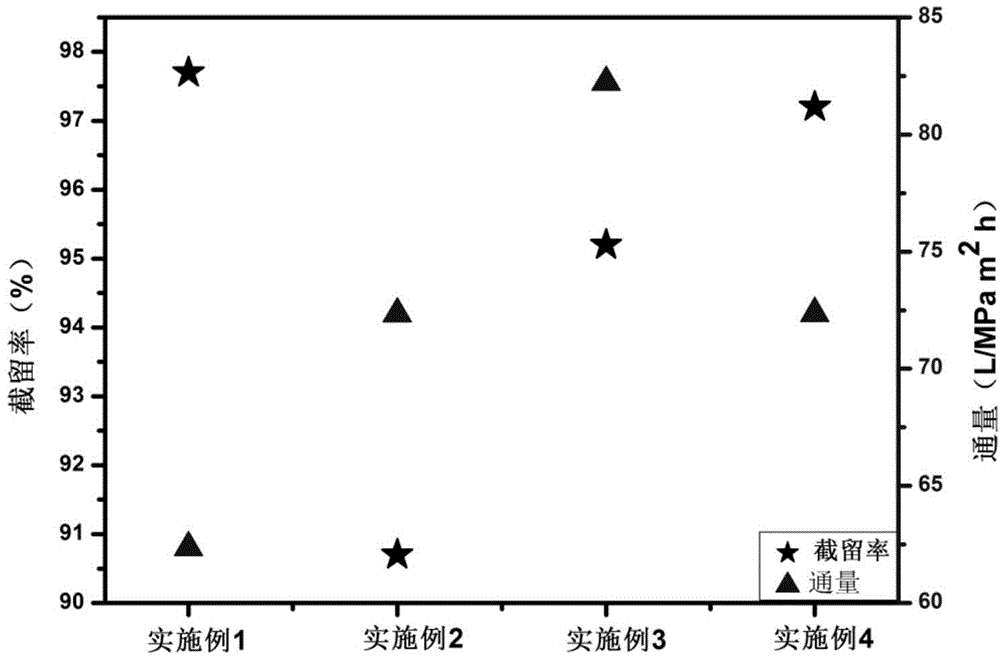
Composite nanofiltration membrane prepared based on aliphatic sulfonate condensation product
ActiveCN103223308ALow costEasy to separateSemi-permeable membranesElectrolysisCationic polyelectrolytes
The invention relates to a composite nanofiltration membrane, especially relates to a composite nanofiltration membrane employing an aliphatic sulfonate condensation product as an anion polyelectrolyte. The composite nanofiltration membrane comprises a basal membrane layer and a composite membrane layer in order, and the composite membrane layer is formed by static self assembly of the cation polyelectrolyte and the anion polyelectrolyte, wherein, the anion polyelectrolyte is an aliphatic sulfonate condensation product solution. The aliphatic sulfonate condensation product is used as an anion polyelectrolyte, and compared with the present anion polyelectrolyte and aliphatic sulfonate condensation product has a lower cost, thereby reducing the cost of the prepared composite nanofiltration membrane, and improving the separating performance for Ni2+, Ca2+ divalent metal ions and xylenol orange, rhodamine B and other dye molecules in heavy metal ion waste water and dye waste water by the prepared composite nanofiltration membrane.
Owner:BEIJING UNIV OF TECH
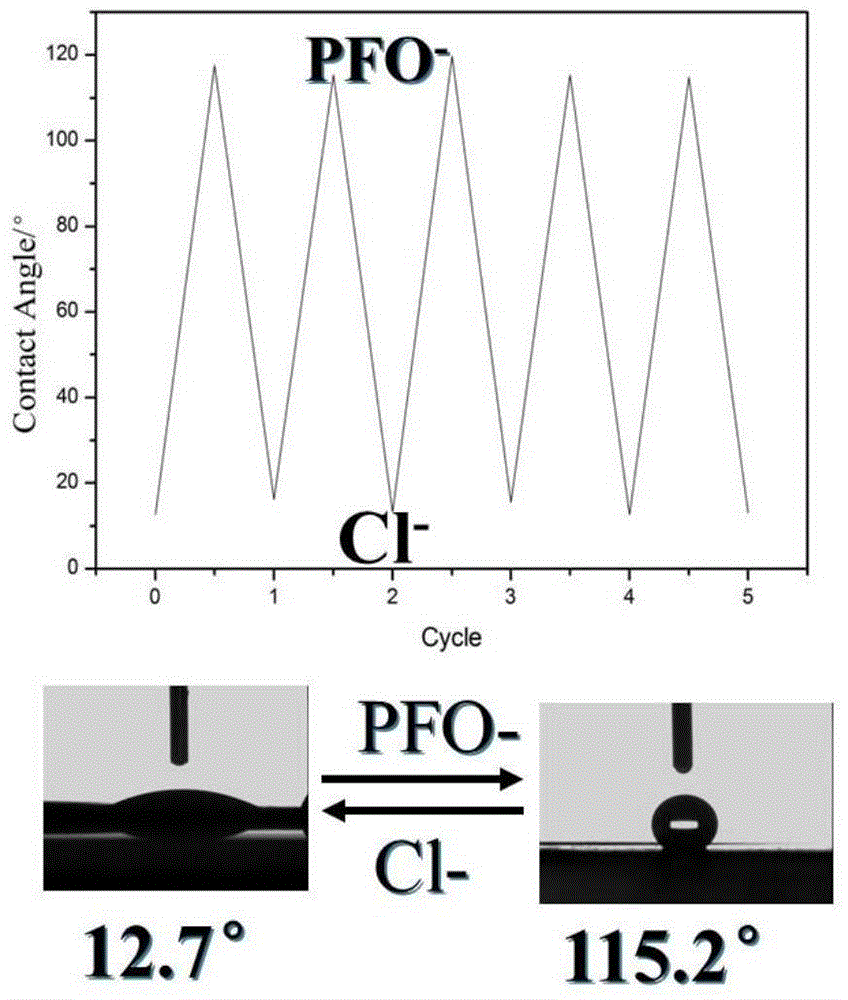
Hydrophilic/hydrophobic transition polyelectrolyte/titanium dioxide composite nanofiltration membrane and preparation method thereof
ActiveCN104923092AAchieve separationEasy to operateSemi-permeable membranesCationic polyelectrolytesUltrafiltration
The invention discloses a hydrophilic / hydrophobic transition polyelectrolyte / titanium dioxide composite nanofiltration membrane and a preparation method thereof, and belongs to the technical field of membranes. According to the key technology, PDDA-TiO2 nano particles, cationic polyelectrolyte and anionic polyelectrolyte are utilized, ultrafiltration membranes such as polysulfone and polyacrylonitrile serve as supporting base membranes, and the composite nanofiltration capable of conducting hydrophilic / hydrophobic intelligent transition is prepared by adopting the method of combining ion exchange and electrostatic layer-by-layer self-assembly. The method is easy to operate and is a green preparation method, and the obtained composite nanofiltration membrane is even in structure; meanwhile, the prepared hydrophilic / hydrophobic transition composite nanofiltration membrane can be used for removing water and dye, such as xylenol orange and methyl blue, of an organic solvent system, and a good application effect is achieved.
Owner:BEIJING UNIV OF TECH
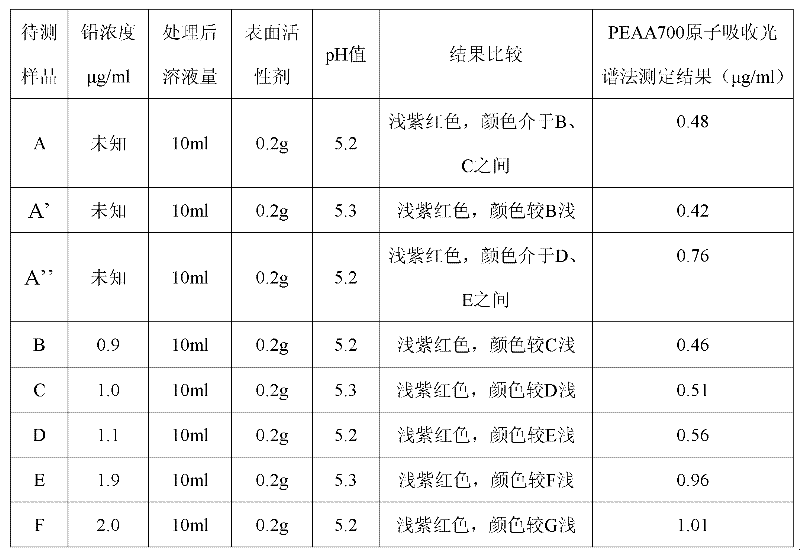
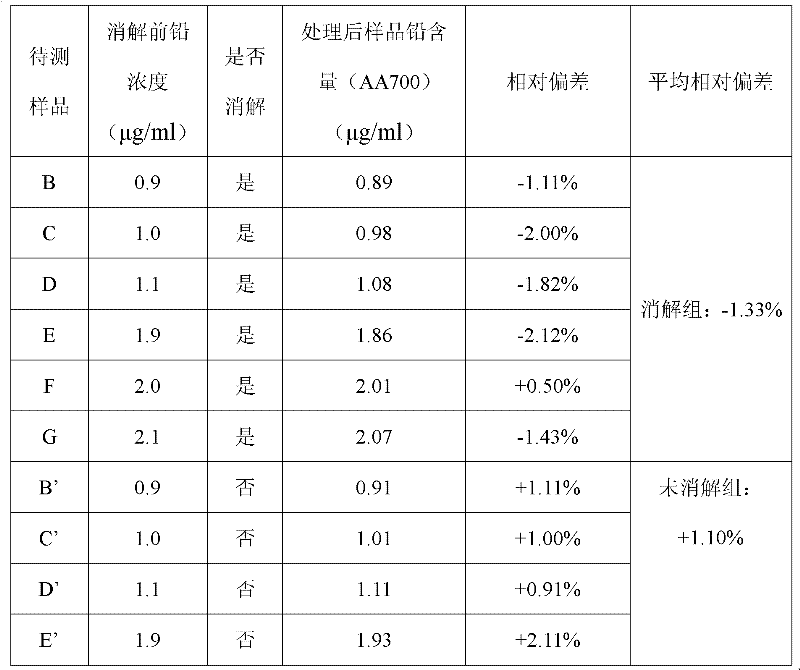
Raid detection method for heavy metal lead in tea leaves
InactiveCN102539425AQuickly determine lead contentDetermine lead contentMaterial analysis by observing effect on chemical indicatorRAIDSurface-active agents
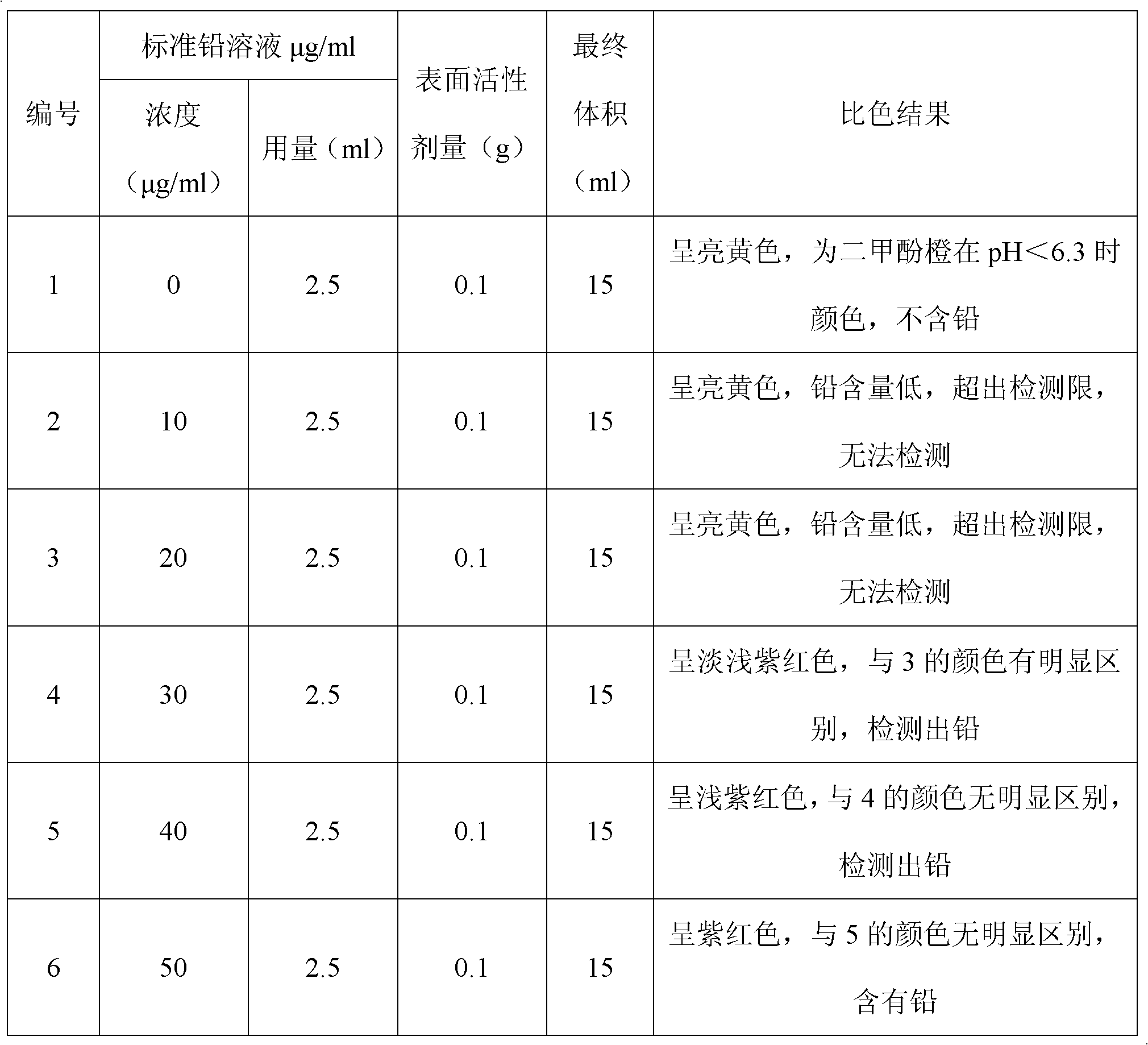
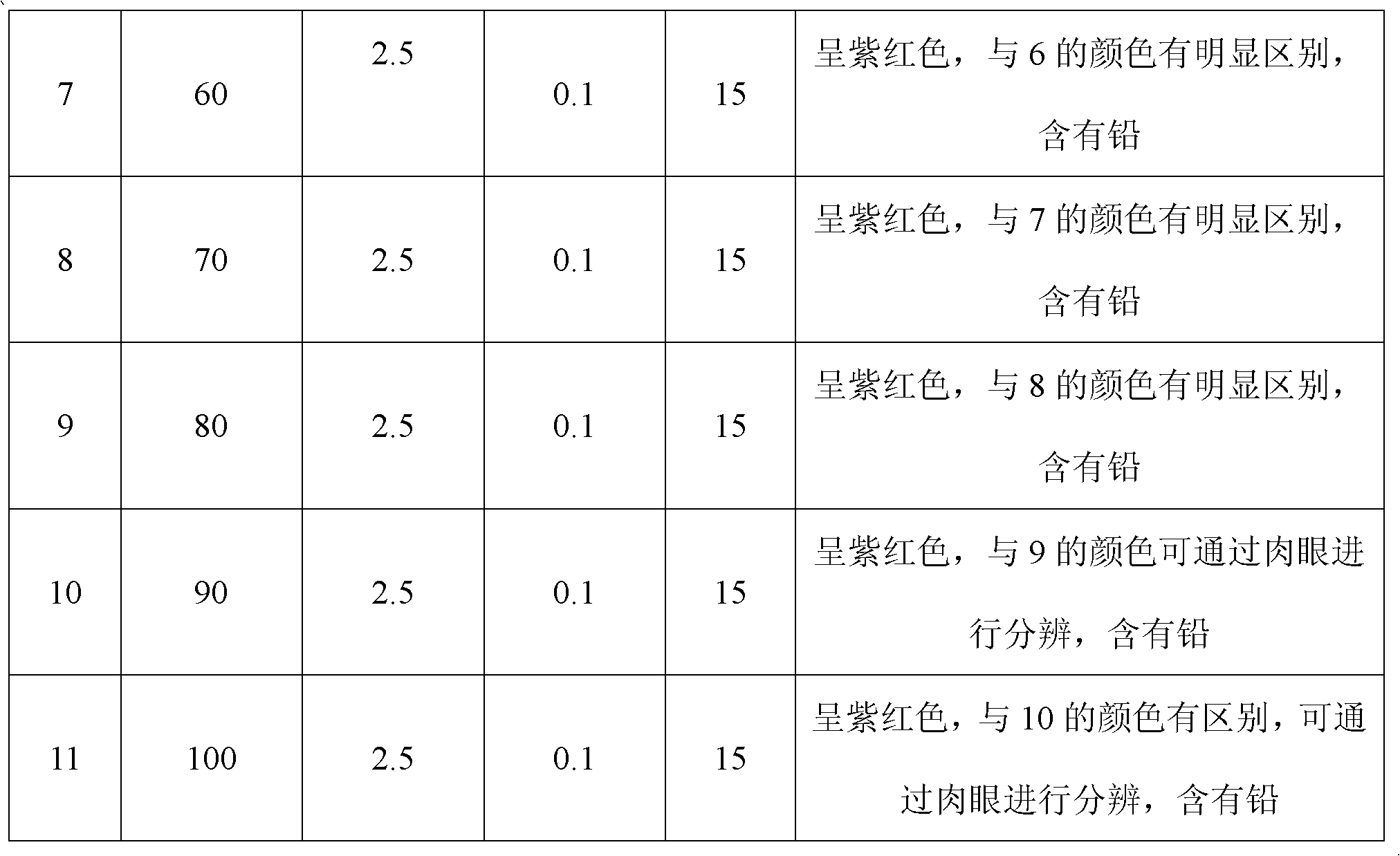
The invention discloses a detection method for heavy metal lead in tea leaves; the detection method comprises the following steps of: taking a crushed tea leaf sample, obtaining a clearly and transparently colorless liquid to be measured through resolution and concentration, adding a polyoxyethylene fatty acid ester surface active agent in the colorless liquid to be measured, and then adding a certain amount of buffer solution and controlling pH to be 4-6, and finally adding a xylenol orange solution with the concentration of 20-30mg / kg in a mixed solution A to obtain a sample solution; taking and processing a standard lead solution with the concentration of 0.90-10mumg / ml according to the same method to obtain a standard solution; and then carrying out color comparison on the sample solution and the stand solution. The detection method can be used for conveniently and rapidly detecting the content of the heavy metal lead in the tea leaves.
Owner:CHINA JILIANG UNIV
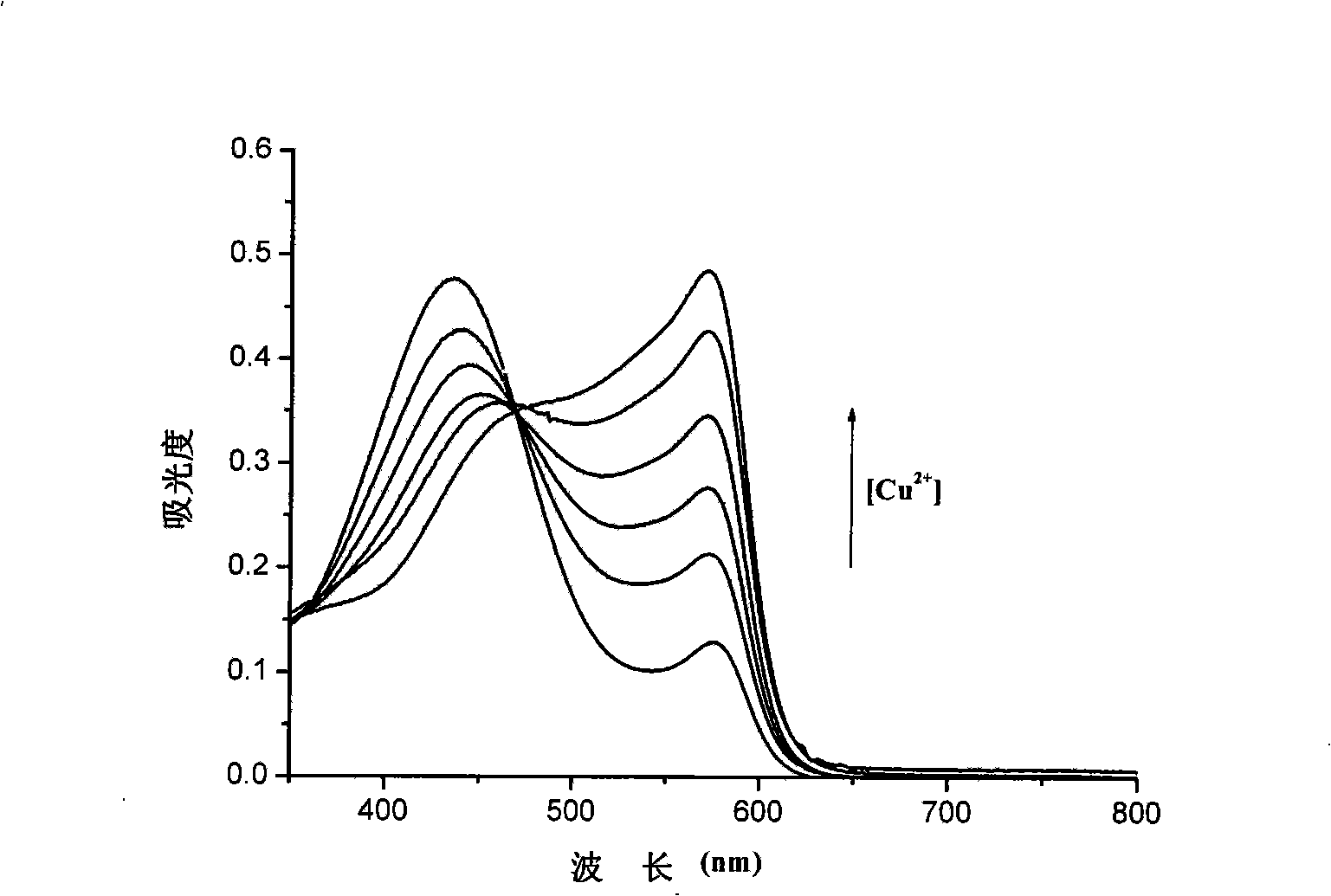
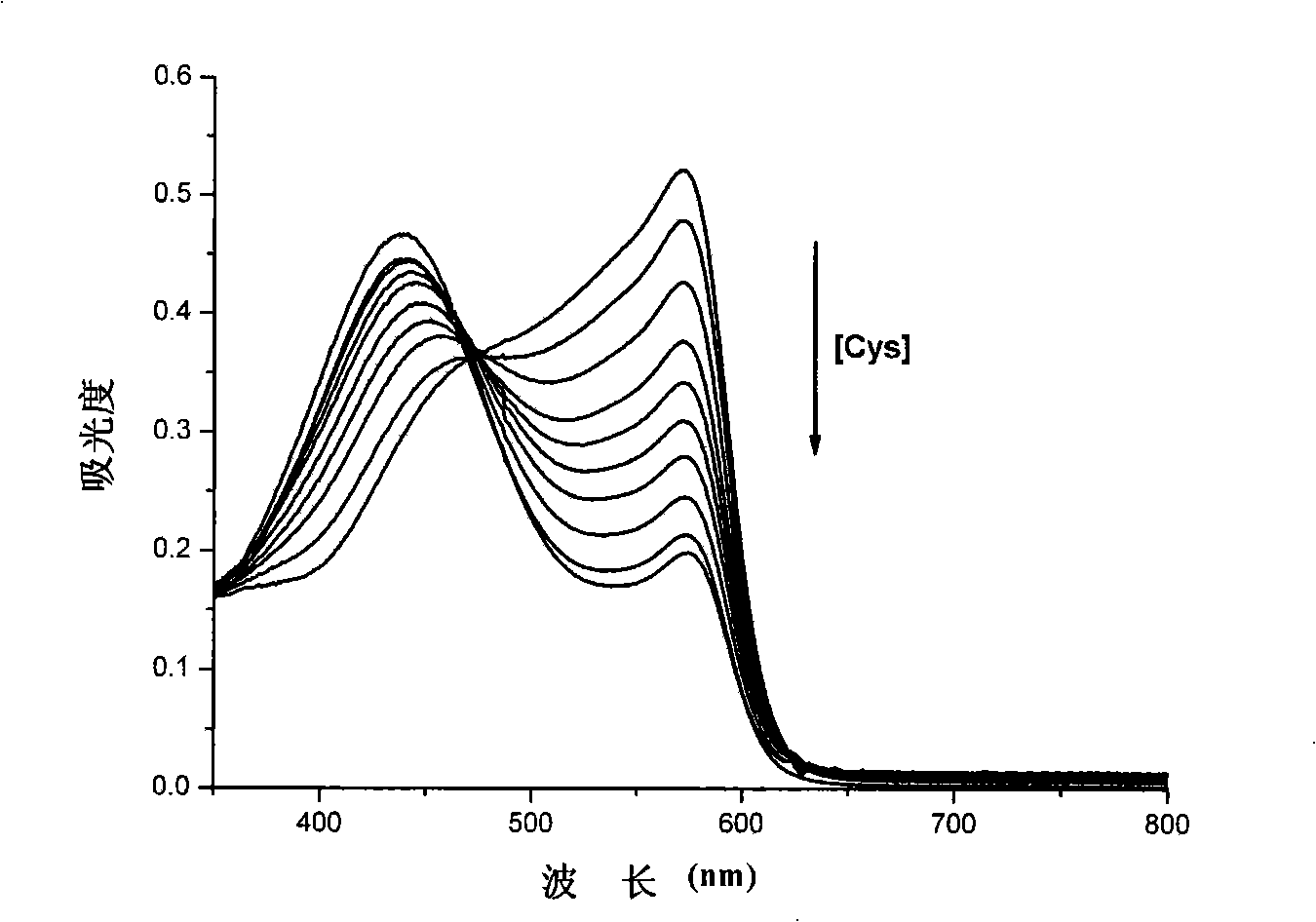
Method for rapidly testing cysteine in water solution
InactiveCN101329279AReduce sensitivityLow selectivityMaterial analysis by observing effect on chemical indicatorBiological testingCupric IonAqueous solution
The invention provides a method for quickly detecting cysteine in a water solution. The method directly detects the content of the cysteine in a buffer solution (the pH value is 6.0) qualitatively and quantitatively by using xylenol orange as a developer on the basis of cupric ion Cu<2+>; the detection process is simple, the judging is objective and convenient and the method of the invention is easy to be generalized and applied. The method of the invention has exact measurement result and high sensitiveness, is not interfered by other aminophenol and negative ions, and can be generalized and applied to the determination of cysteine in clinical blood samples, urine samples and food.
Owner:SHANXI UNIV
Method for measuring free lead in lead-acid battery green plate sample
InactiveCN102426168ASimplified assay method stepsReduce lossesMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationEthylene diamineTetramine
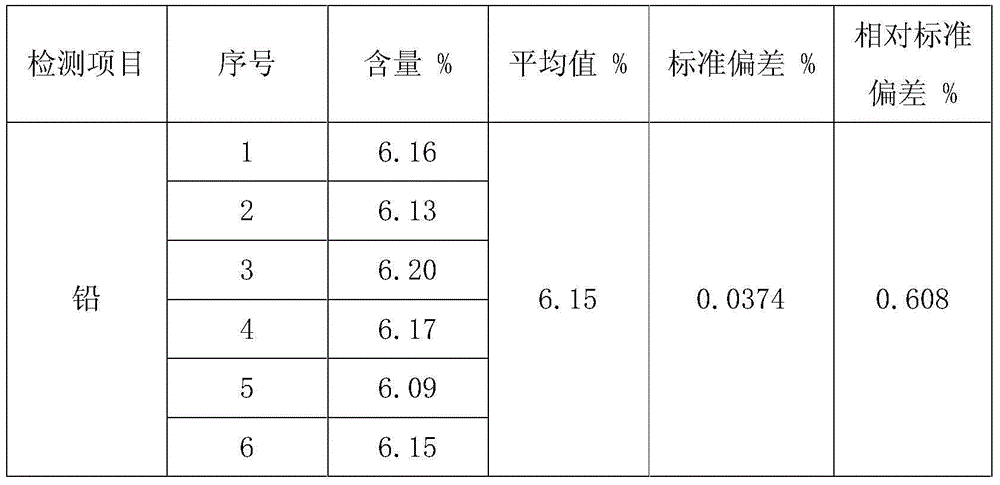
The invention discloses a method for measuring free lead in a lead-acid battery green plate sample. The method comprises: conducting sampling from a green plate, carrying out weighing, and adding the sample into an ammonium acetate solution till fully dissolved, removing the clear liquid, then adding the obtained product into a nitric acid solution till fully dissolved, adjusting the pH of the solution, adding a hexamethylene tetramine solution, dripping a xylenol orange indicating liquid into the mixed solution, and shaking it well, performing titration with an EDTA (ethylene diamine tetraacetic acid) solution till the solution turns from purple red into bright yellow, taking down the volume of the used EDTA solution, and calculating the titer of the EDTA solution to Pb (plumbum) according to the concentration of the EDTA solution, then calculating the Pb content in the original sample. The method of the invention simplifies the steps of a free lead measuring method, reduces the reagents consumed, and lowers the loss of the target object free lead.
Owner:CHONGQING WANLI NEW ENERGY CO LTD
Analysis method for quickly and accurately measuring zinc in zinc electrolyte
InactiveCN102645432AInfluence of Shielding Zinc DeterminationSolve the problem of inaccurate and reliable analysis dataMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationSodium acetateElectrolytic agent
The invention provides an analysis method for quickly and accurately measuring zinc in zinc electrolyte. The method comprises the following steps of: weighing quantitative zinc electrolyte in a container, blowing water, then adding excessive ammonium chloride, and shaking uniformly; adding excessive ammonia water and hydrogen peroxide, and shaking uniformly; boiling until no continuous air bubbles are generated; breaking the hydrogen peroxide, and performing hot filtration with quick filter paper; washing the filtering container with ammonium chloride-ammonia water washing liquid, and precipitating; uniformly shaking the filtrate, and heating for concentration; cooling and adding ascorbic acid; shaking uniformly and adding a xylenol orange indicator; adjusting with hydrochloric acid and ammonia water until the solution is yellow; adding saturated sodium fluoride and thiourea and shaking uniformly; adding acetic acid-sodium acetate buffer solution, and shaking uniformly; and titrating with the EDTA (Ethylene Diamine Tetraacetic Acid) standard titration solution until the solution becomes bright yellow from purple red. Through the invention, the influence of manganese ion on the measurement of zinc content in zinc electrolyte can be effectively shielded, the problem that the analysis data is not accurate or reliable in the existing method is solved, and the measured result has an instruction effect on the production of zinc electrolyte.
Owner:BAIYIN NONFERROUS GROUP
Radiating color changing hydrogels three-dimensional monitor and method for making same
InactiveCN101387706ASimple and fast operationNo pollution in the processChemical dosimetersResponse sensitivitySide effect
The invention relates to a novel radiation discoloration hydrogel three-dimension dose meter and a preparation method thereof, wherein the hydrogel three-dimension dose meter is formed by mixing a polyvinyl alcohol PVA acid water solution and dose meter gel beads in a mass ratio of 20: (1-3). The invention utilizes freeze and thaw physical crosslink method to prepare the hydrogel dose meter, which has simple operation and has no pollution or toxic side effect, compared with chemical crosslink method. Under radiation, the Fe2+ of the hydrogel three-dimension dose meter is oxidized into Fe3+, and fits the xylenol orange (X0) to form a color development XO-Fe3+ complex, thereby obtaining significant color gradient change to visually observe the distribution of the radiation dose. The invention utilizes liquid nitrogen quickly freeze to prepare and disperse dose meter gel beads in a PVA substrate, thereby simulating cell independence in a certain degree, to improve the dose response sensitivity and the stability after radiation.
Owner:SHANGHAI UNIV
Method for measuring tin element by EDTA (Ethylene Diamine Tetraacetic Acid) complexation
ActiveCN102053088ALow costGuaranteed measurement accuracyMaterial analysis by observing effect on chemical indicatorRelative standard deviationOxidative treatment
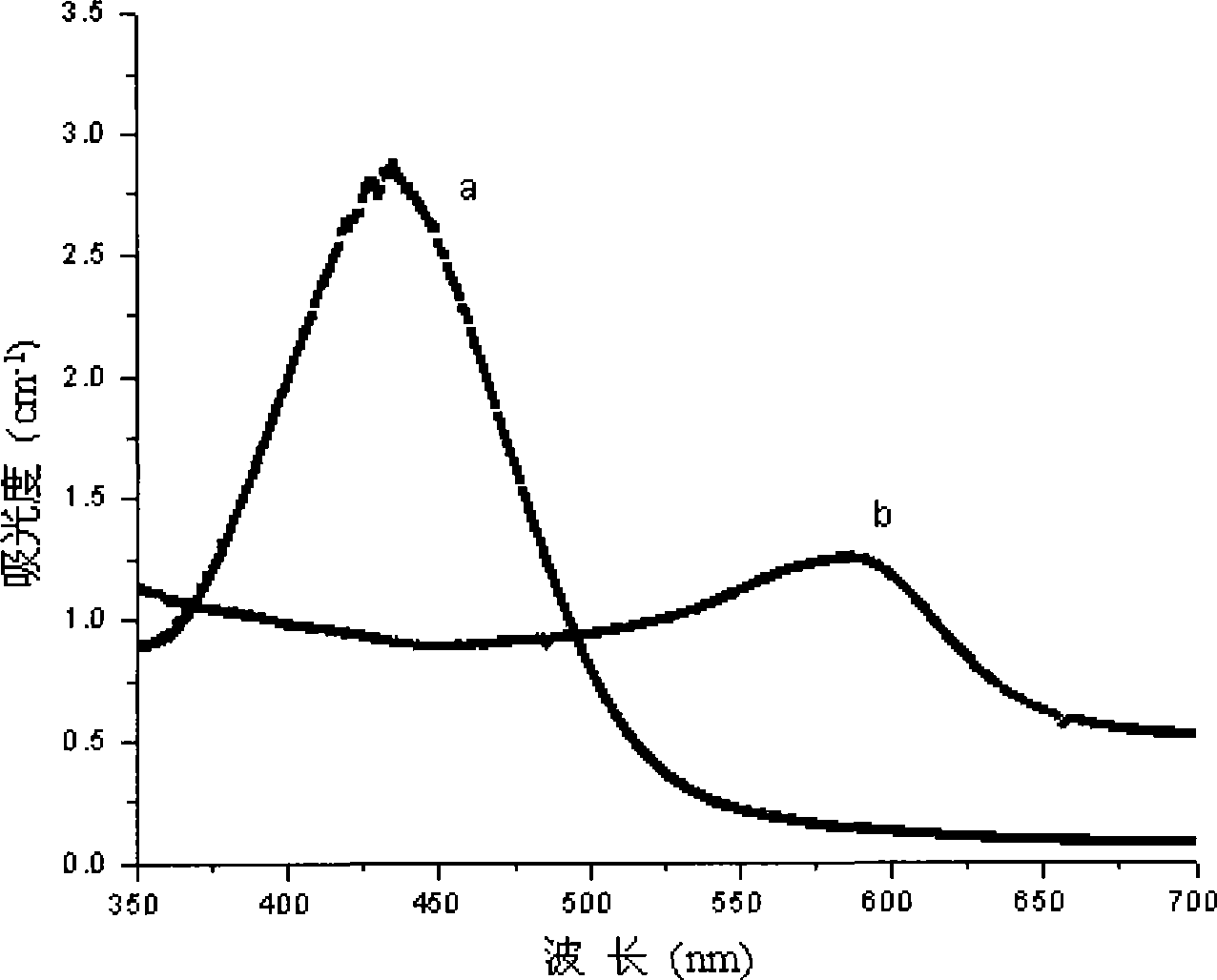
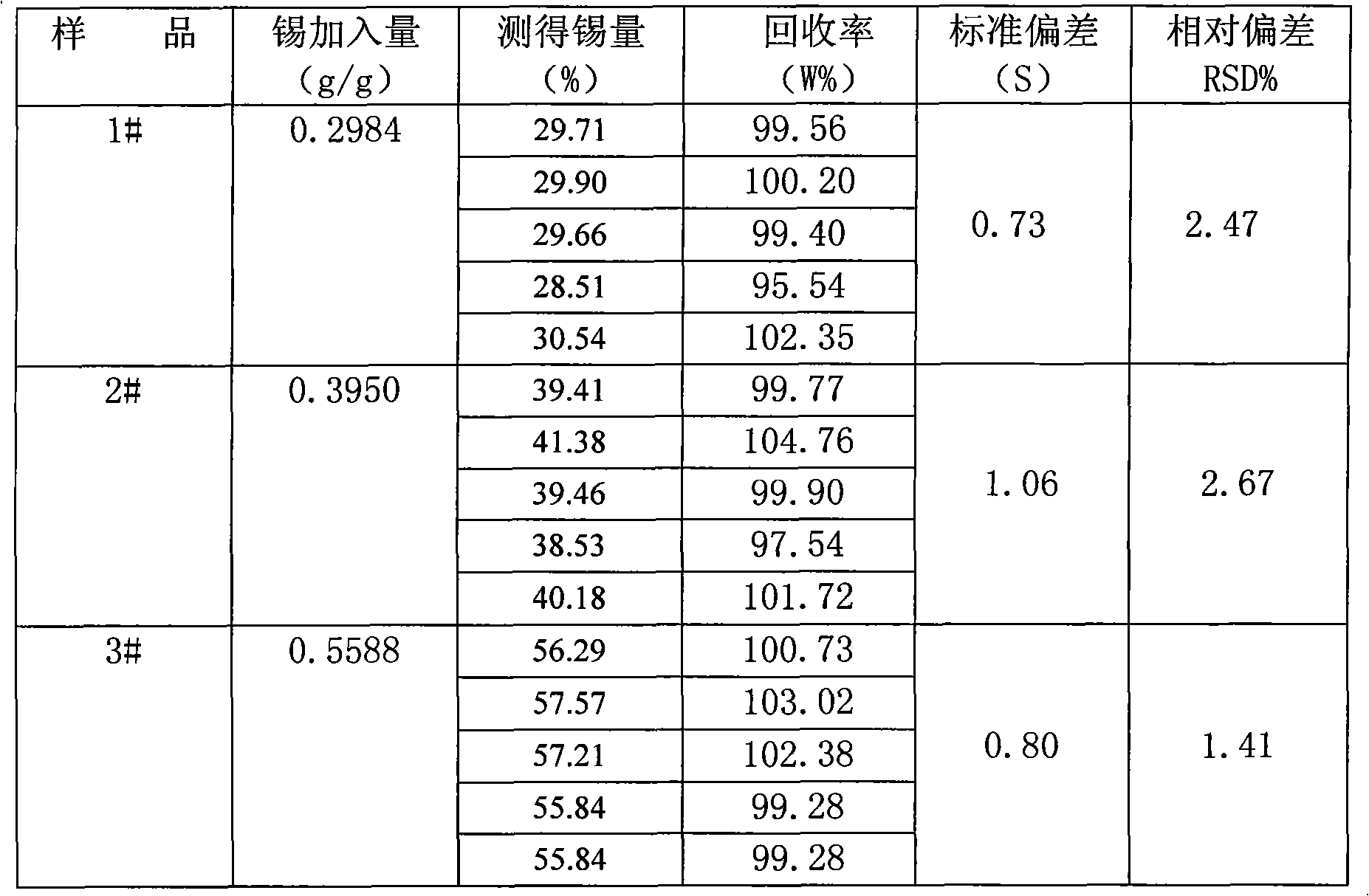
The invention relates to a method for measuring tin elements, comprising the following steps of: deoxidating a sample; dissolving with the mixed acid of hydrochloric acid and nitric acid; adjusting acidity of the solution with ammonia water and the hydrochloric acid; quantificationally complexing the tin and other elements capable of complexing with EDTA with excessive EDTA; with xylenol orange as an indicator, titrating excessive EDTA with the standard titration solution; buffering with hexamethylene tetramine to ensure that the pH value is between 5 and 6; adding sodium fluoride for separating out EDTA which is equivalent to the amount of the tin; titrating separated EDTA with the standard titration solution until the solution becomes red from yellow; and calculating the percentage of the tin according to the volume of the standard titration solution consumed in the second titration. The method has the recovery rate of 95.54-104.76% and the relative standard deviation of 1.41-2.67%. The reagents used in the method are all common chemical reagents, and no special devices are needed. In addition, the method has the advantages of simple process, short measurement period and low cost.
Owner:SHAANXI AIRCRAFT CORPORATION
Determining method for lead contents in gold concentrate and lead concentrate
InactiveCN105842390ASolve complexityAddressing differences in lead contentChemical analysis using titrationSodium acetateLead sulfate
The invention relates to a determining method for lead contents in gold concentrate and lead concentrate and belongs to a determining method for lead contents in ore and concentrate. The determining method comprises the following steps of using nitric acid, bromine water and sulfuric acid to digest a sample, and using hydrobromic acid and dilute sulphuric acid to treat for removing the interference of elements such as arsenic, antimony, selenium and tin in the sample to lead determination; then adopting sulphuric acid and lead to form lead sulfate precipitation, and filtering and separating out other interference elements; transferring the precipitation and filter paper to an acetic acid-sodium acetate buffer solution, enabling a mixture to generate lead acetate and dissolving in the buffer solution; taking xylenol orange as an indicator, and using a Na2EDTA standard titration solution for titrating. The accuracy, the precision and the reproducibility of the method disclosed by the invention can completely reach the determination requirement of the lead contents; meanwhile, the method has the advantages of simplicity and convenience in operation and high efficiency, and has important significance for quality monitoring, metal balance and trade pricing.
Owner:CHANGCHUN GOLD RES INST
Determination method of zinc in gold alloy
ActiveCN106198854AAccurate determination of contentSuitable for large batch analysisChemical analysis using titrationColor/spectral properties measurementsSodium acetateHydrazine compound
The invention relates to a determination method of zinc in gold alloy, and belongs to a determination method of amount of zinc in gold alloy. The method comprises the following steps: aqua regia is used for dissolving a sample material, hydrazine hydrate is used for deposition and separation gold, filtering is carried out, and filter residues are used for recycling gold; sodium hydroxide and a hydrogen peroxide solution are added into a filtrate for deposition and separation of nickel and copper, filtering is carried out, and an acetic acid-sodium acetate buffer solution is added into the filtrate; xylenol orange is used as an indicator, EDTA is used for titration till the color of the solution changes into luminous yellow from wine red, and the titration volume is used for calculating the content of zinc; hydrochloric acid is added into the filter residue for dissolving, volumetric flask is prepared, the amount of zinc is determined by an atomic absorption spectrometer at the wavelength of 213.8nm, and sum of the zinc contents which are determined by the two determination methods is the content of the zinc in the alloy. The method can be used for accurately determining the content of zinc in the gold alloy, and is suitable for analysis on a large scale, at the same time the method fills the blank of determination methods of zinc in gold alloy.
Owner:SHENZHEN JINZHI GOLD&SILVER JEWELLERY INSPECTION RES CENT CO LTD
Chemical analysis method for determination of zirconium content in alloy containing various interference elements
InactiveCN103616472AResolve interferenceResolve separabilityChemical analysis using titrationAlloySodium hydroxide
The invention discloses a chemical analysis method for the determination of the zirconium content in an alloy containing various interference elements. The chemical analysis method is characterized by comprising the following steps: adding a nitric acid, a hydrofluoric acid and a sulfuric acid into a platinum dish to dissolve an alloy specimen, and separating interference elements by using sodium hydroxide; titrating zirconium in precipitations by using EDTA (ethylene diamine tetraacetic acid) by taking xylenol orange as an indicator in a hydrochloric acid medium with a hydrogen ion molar concentration of 1.25 moles per liter at a temperature of 90-100 DEG C. The method disclosed by the invention has the advantages that the method is especially applicable to the determination of the zirconium content of a drilling-burning double-function alloy, the determination results are accurate, and the determination process is convenient.
Owner:CHINA WEAPON SCI ACADEMY NINGBO BRANCH +1
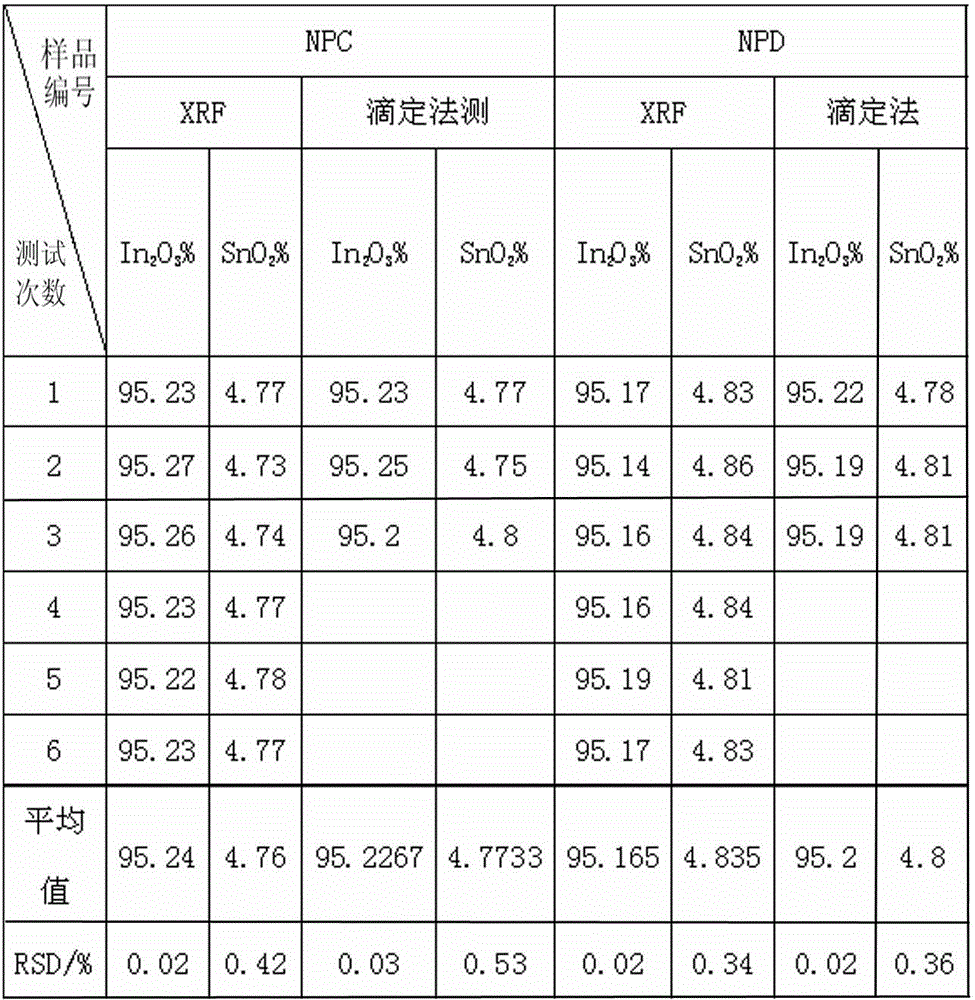
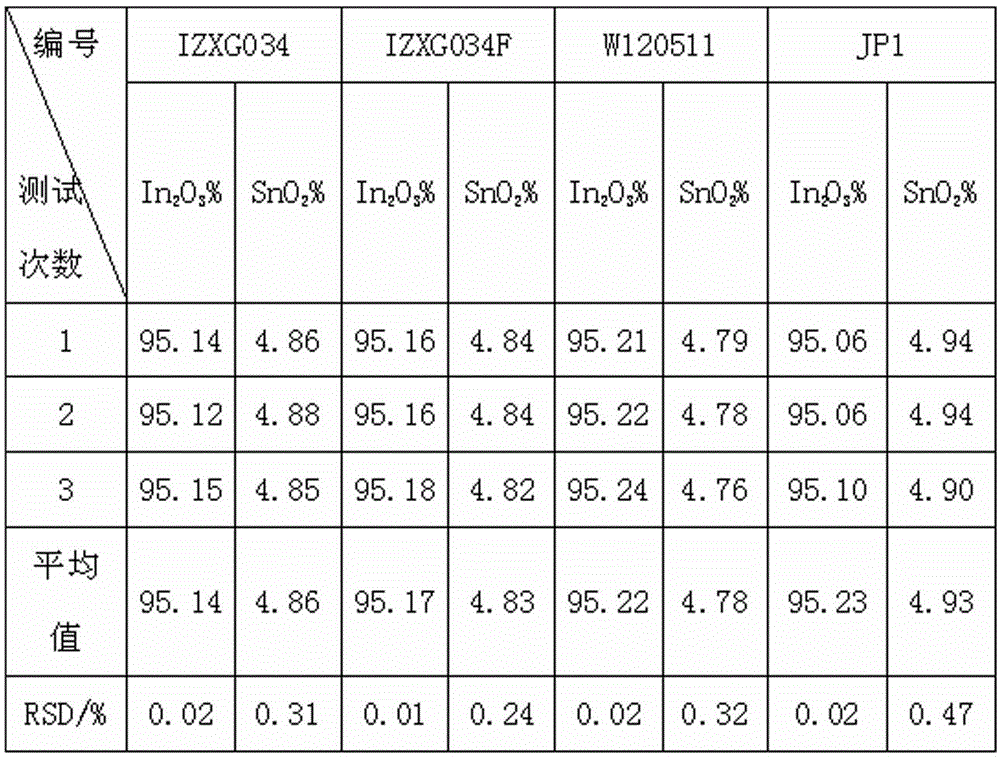
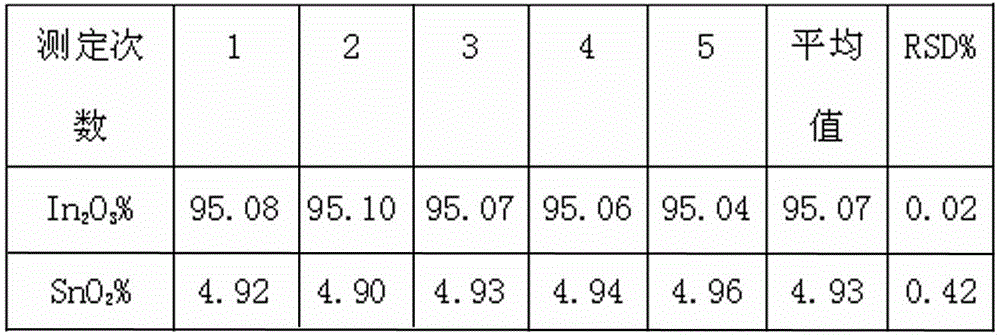
Indium tin oxide (ITO) target material and determination method for content of indium and tin during production process of ITO target material
InactiveCN102721688AEasy to operateImprove accuracyMaterial analysis by observing effect on chemical indicatorEthylene diaminePotassium nitrate
The invention relates to an indium tin oxide (ITO) target material and a determination method for content of indium and tin during a production process of the ITO target material, after a sample is dissolved by acid, potassium nitrate is added to liquid to be determined, excessive ethylene diamine tetraacetic acid (EDTA) is subjected to complexation with indium and tin, ammonia water is used for adjusting pH value, the solution is heated to a boiling state, and cooled to a room temperature; hexamethylene tetramine is added for adjusting pH value, indicator xylenol orange is added, titration of the solution is carried out by zinc standard liquid until the color is changed from yellow to red, and the total content of the indium and the tin in the sample is converted through a consumption volume of the zinc standard liquid; and ammonium fluoride masking tin is added, after ammonium fluoride is completely dissolved, the solution is heated to a requested temperature and cooled to the room temperature, and the solution presents yellow, finally the titration of the solution is carried out by the zinc standard liquid until the color becomes red which is a titration finishing point, and the content of indium oxide in the sample is converted through the consumption volume of the zinc standard liquid. According to the ITO target material and the determination method, continuous titration of the indium and the tin can be realized, the titration efficiency is high, and the test result has remarkable accuracy, stability and repeatability.
Owner:SHAOGUAN SIGMA TECH
A method for measuring nickel in raw gold
InactiveCN107478765AAccurate measurementEasy to recycleChemical analysis using titrationHexamethylenetetramineFiltration
The invention relates to a method for measuring nickel in raw gold, and belongs to methods for measuring the nickel content in raw gold. The method includes dissolving a specimen with nitrohydrochloric acid; precipitating and separating gold by utilizing hydrazine hydrate; performing filtration with the filter residue being used for recovering the gold; adding ammonium chloride and ammonia water into the filtrate to form an alkaline buffer system; precipitating and separating nickel by adding dimethylglyoxime into the system; adding an excessive amount of EDTA and reacting the EDTA with the nickel by adopting hexamethylenetetramine as a buffer solution; titrating the excess EDTA until an end point of a color conversion from yellow to purplish red of the solution by adopting xylenol orange as an indicator and adopting zinc chloride as a titrant; and calculating the nickel content of the raw gold. The method can accurately measure the nickel content of the raw gold, is suitable for large-scale analysis, and fills the gap of nickel content measurement methods for raw gold.
Owner:CHANGCHUN GOLD RES INST
Rapid analysis method of lead in gold mud
ActiveCN105044097AFill vacancyEliminate distractionsMaterial analysis by observing effect on chemical indicatorSodium acetateSodium acetrizoate
The invention relates to a rapid analysis method of lead in gold mud. The method comprises the following steps: processing a sample by nitric acid, aqua regia, sulfuric acid, and a mixed acid of nitric acid and sulfuric acid in sequence, after the acid is smoked and evaporated, adding diluted sulfuric acid, boiling to dissolve the salts, adding excess sodium hyposulfite to reduce gold to zero valent and precipitate silver; allowing the system to stand still to carry out aging, filtering, washing the precipitate by diluted sulfuric acid and water; dissolving the precipitate by an acetic acid-sodium acetate buffer solution, filtering, recovering gold and silver, adding a xylenol orange indicator into the filtrate, dropwise adding EDTA until the color of the solution changes from purple-red to light yellow, wherein the color change shows that the titration end point is reached, and finally calculating the lead content. In the provided method, sodium hyposulfite is used to reduce gold and precipitate silver so as to effectively separated gold and silver from lead, thus the interference of gold and silver on the end point is avoided, moreover, the operation is largely simplified, and precise and stable analysis results can be obtained by the provided method.
Owner:山东黄金冶炼有限公司
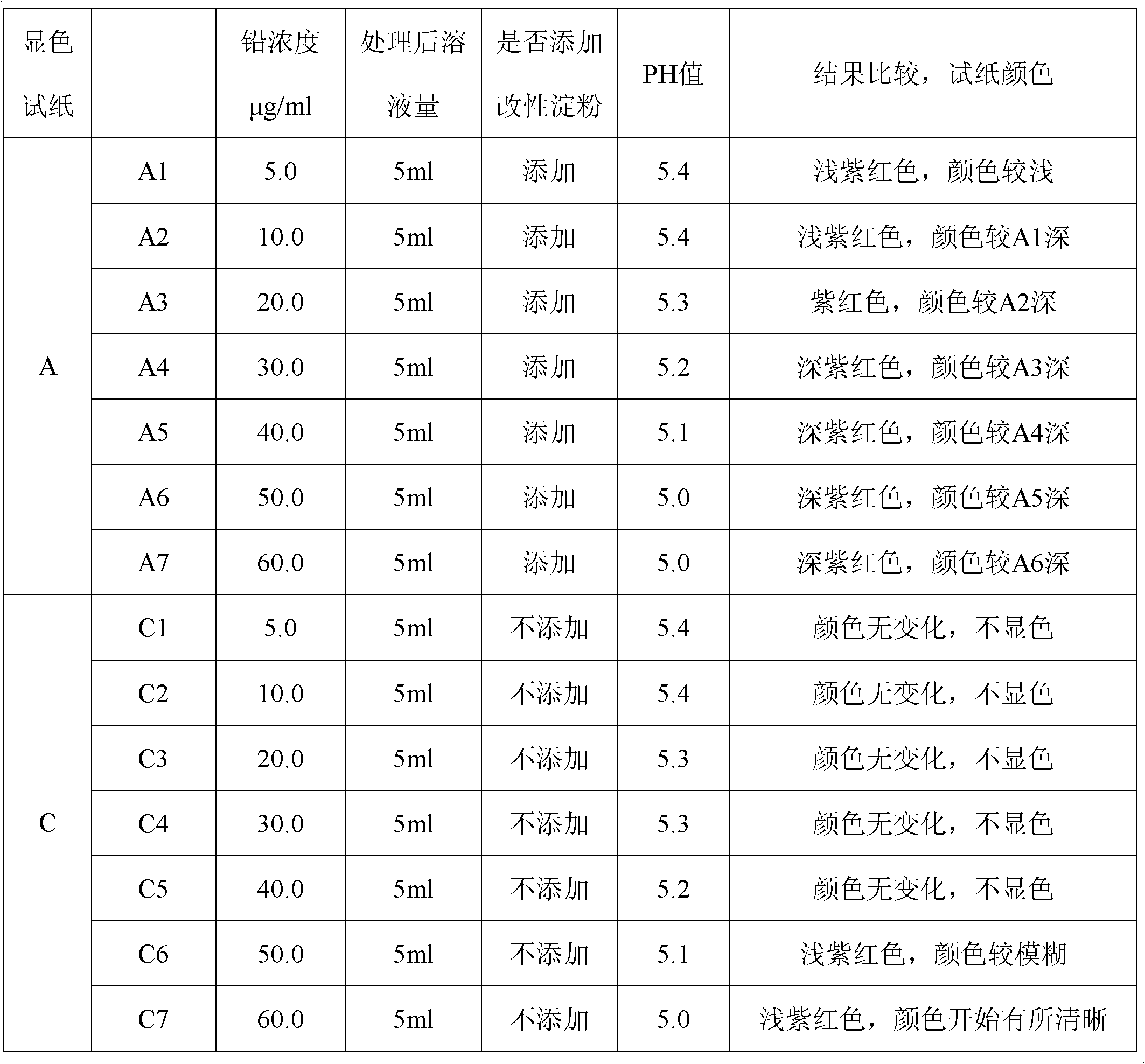
Qualitative and semiquantitative lead detection test paper and application thereof
InactiveCN102565051AImprove detection accuracyQuick monitoringMaterial analysis by observing effect on chemical indicatorEthylene diamineLow speed
The invention discloses qualitative lead detection test paper and semiquantitative lead detection test paper and application thereof. The qualitative lead detection test paper comprises developing test paper A which is obtained by the steps of soaking low-speed quantitative filter paper in a developer solution A, taking the low-speed quantitative filter paper out, and drying the low-speed quantitative filter paper at low temperature; the developer solution A is prepared from xylenol orange, thiourea, aminothiopropionic acid, modified starch and deionized water; and in the developer solution A, the concentration of the xylenol orange is 30 to 50mg / kg, the concentration of thiourea is 1 to 10mg / kg, the concentration of the aminothiopropionic acid is 1 to 10mg / kg, and the modified starch is 1 to 5mg / kg. The difference between the semiquantitative lead detection test paper and the qualitative lead detection test paper is that: a developer solution B which is used by the semiquantitative lead detection test paper contains ethylene diamine tetraacetic acid disodium salt at the concentration of 23.43mg / L except substances contained in the developer solution A. The qualitative lead detection test paper and the semiquantitative lead detection test paper can be used for qualitative and semiquantitative detection of lead in water cosmetics.
Owner:CHINA JILIANG UNIV
Chemical analysis method for lead elements in tin-lead alloy solder
InactiveCN102507571ALow costHigh measurement accuracyMaterial analysis by observing effect on chemical indicatorPotassium sodium tartrateAlloy
The invention provides a chemical analysis method for lead elements in a tin-lead alloy solder. The chemical analysis method comprises the following steps of: A, accurately weighing a certain amount of tin-lead alloy solder samples into a beaker, adding nitric acid into the beaker, and putting the beaker on a heating plate for heating; after producing white smoke, adding hydrogen peroxide into the beaker for continuously heating until reaction is finished, adding hydrochloric acid for continuously heating until the samples are completely dissolved; B, adding a tartaric acid potassium sodium solution, deionized water and triethanolamine into the beaker after cooling; using a hexamethylene tetramine-ammonia water mixture to regulate the PH value of a test solution within the range of 5.5-6.0, dripping a xylenol orange indicator, and using an EDTA (Ethylene Diamine Tetraacetic Acid) standard titration solution for titrating until the mixture is stable luminous yellow; C, during test, carrying out a blank test; and D, calculating according to a formula to obtain the mass fraction of the lead elements in the tin-lead alloy solder. The method brings the beneficial effects that according to the method, the cost of measurement and analysis is effectively reduced, and the measurement accuracy is increased.
Owner:苏州华碧微科检测技术有限公司
pH nanometer sensing material with upconversion luminescence property, and preparation method thereof
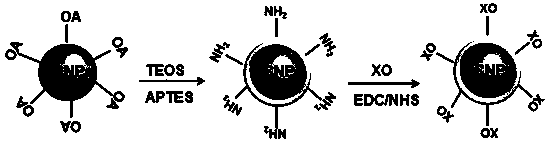
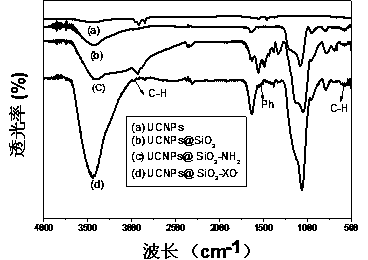
InactiveCN103468260ASmall particle sizeUniform particle sizeMaterial nanotechnologyNanoopticsOrganic dyeBiocompatibility
The present invention relates to a pH nanometer sensing material with upconversion luminescence property, and a preparation method thereof. According to the present invention, a fluoride is adopted as a matrix, and is doped with trivalent rare earth ions to obtain a rare earth upconversion luminescence nanometer crystal, in order to broaden applications of the rare earth upconversion luminescence nanometer crystal in the organism environment, surface modification is performed on the nanometer crystal from hydrophilicity / hydrophobicity of a surface ligand, ligand electric charge, coordination capacity and other angles, silica coating is performed on the surface of the nanometer crystal to functionalize the amino group, and the obtained material and an organic dye xylenol orange are conjugated to obtain a composite nanometer crystal, wherein the size is 44-80 nm, and the composite nanometer crystal can be well dispersed in water and can be stable in aqueous solutions. In addition, the composite nanometer crystal has characteristics of controllable size, uniform particle size and good biocompatibility, and can be used for detection of solution pH values and pH values in living cells.
Owner:NANJING UNIV OF POSTS & TELECOMM
Method for testing fluorine content in solution
InactiveCN104198484ANo pollution in the processSimple methodMaterial analysis by observing effect on chemical indicatorSodium acetateIon content
The invention relates to a method for testing fluorine content in a solution. The adopted the technical scheme comprises the following steps: adding deionized water into a conical flask, then accurately transferring a fluorine ion solution to be tested into the conical flask, shaking fully uniformly, adding potassium nitrate, accurately transferring a standard lanthanum nitrate solution into the conical flask, uniformly mixing, regulating the pH of the solution to be 1.0-3.0, heating and boiling for 1-2 minutes, and cooling to the room temperature; adding 10mL of acetate-sodium acetate buffered solution and five to six drops of xylenol orange indicator, and dripping into an EDTA (Ethylene Diamine Tetraacetic Acid) standard solution until the solution turns bright yellow from prunosus. The method is simple and environment-friendly and can test the fluorine ion content in the fluoride solution of which the fluorine ion concentration is greater than 0.1mg / mL.
Owner:SHENYANG AIRCRAFT CORP
Method for measuring contents of bismuth and lead ions in tin lead bismuth alloy electroplating solution
InactiveCN105021545AThe determination method is simpleEasy to detectMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationHexamethylenetetramineThiourea
The invention relates to a method for measuring contents of bismuth and lead ions in a tin lead bismuth alloy electroplating solution. According to the method, bismuth and thiourea react in 0.4 mol / L-1.2 mol / L of a nitric acid medium to generate a soluble yellow complex, interference of lead is eliminated with tartaric acid, interference of ferric ions is eliminated with ascorbic acid, and the content of bismuth in an electroplating solution is measured by a spectrophotometric method; and Sn<2+> is oxidized into Sn<4+> by the use of hydrogen peroxide in an acid solution, excessive EDTA-2Na complex bismuth, lead and the like are added, tin is screened with ammonium fluoride, a hexamethylenetetramine solution is added to adjust pH value of the solution to 5-6, xylenol orange is used as an indicator, back titration is carried out with a zinc standard titration solution, the content of lead in the electroplating solution is calculated according to the quantity of EDTA-2Na and the zinc standard titration solution, and determination of bismuth interference with lead is deducted during the calculation. The determination method is simpler, the detection process is faster, more convenient and more environmentally-friendly, expense cost is lower, and detection results are more accurate.
Owner:江南工业集团有限公司
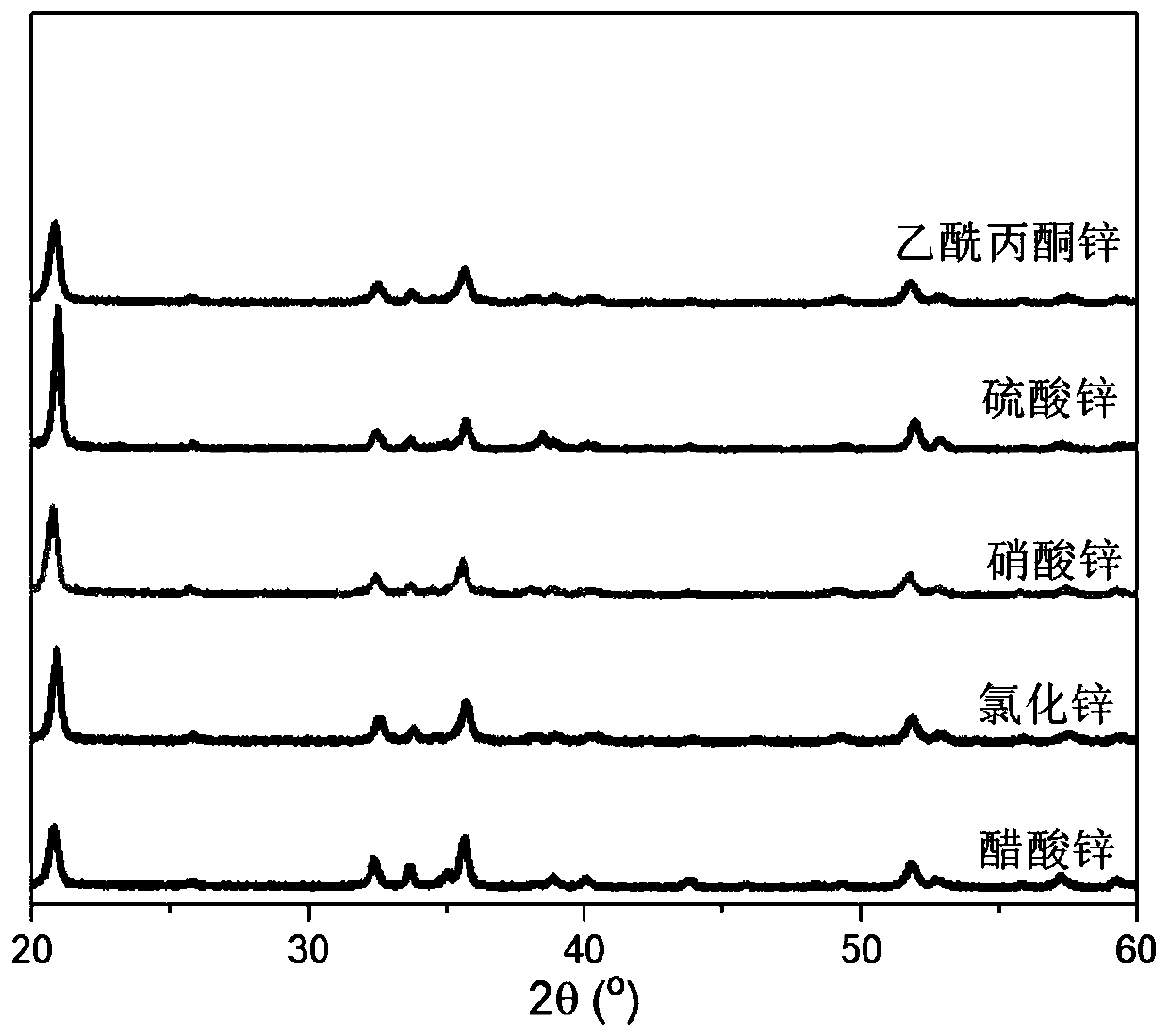
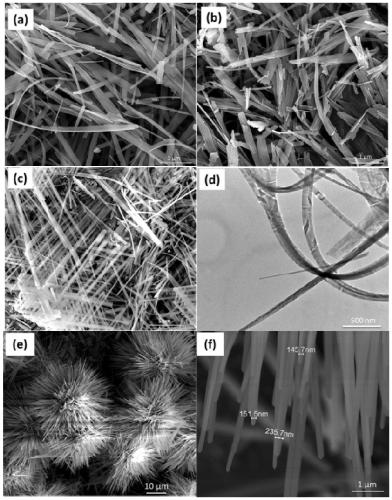
Controllable synthesizing method for hydroxy zinc fluoride nanometer materials with different morphologies and environmental photocatalytic application of hydroxy zinc fluoride nanometer materials
ActiveCN109970098AThe shape features are rich and diverseThe shape features are rich and diverse and can be adjustedZinc halidesPhysical/chemical process catalystsBenzeneCresol
The invention discloses a controllable synthesizing method for hydroxy zinc fluoride nanometer materials with different morphologies and environmental photocatalytic application of the hydroxy zinc fluoride nanometer materials. A hydrothermal reaction is adopted, by controlling a zinc source, a solvent, alkali and the temperature and time of the hydrothermal reaction, the morphologies of the hydroxy zinc fluoride nanometer materials are controlled, and the hydroxy zinc fluoride nanometer materials with different morphologies are obtained. The preparation method is simple, the reaction conditions are mild, and the yield is high. The morphologies of the hydroxy zinc fluoride nanometer materials obtained by using the method are rich, varied and controllable, and the hydroxy zinc fluoride nanometer materials are obvious in catalytic activity and capable of being used for photocatalytic degradation of various organic pollutants, such as brilliant cresol blue, rhodamine B, xylenol orange, bromocresol green, Congo red, methylene blue, phenol and benzene.
Owner:HUAIBEI NORMAL UNIVERSITY
Inductively coupled plasma-atomic emission spectrometry for measuring chromium and zirconium in Cu-Cr-Zr alloy
InactiveCN104614365AQuick checkEasy to operateAnalysis by thermal excitationRelative standard deviationZirconium alloy
The invention discloses an inductively coupled plasma-atomic emission spectrometry for measuring chromium and zirconium in a Cu-Cr-Zr alloy. According to the invention, nitric acid and sulfuric acid are used to dissolve a sample, spectral lines at 284.984nm and at 343.823nm without interference among elements are selected as analytical lines of Cr and Zr, a copper matrix matching technology is adopted, and chromium and zirconium in a Cu-Cr-Zr alloy are measured by an inductively coupled plasma-atomic emission spectrometry (ICP-AES). Detection limits of chromium and zirconium are respectively 0.009 microgram / mL and 0.012 microgram / mL. The above method is used for measuring chromium and zirconium in the Cu-Cr-Zr alloy sample. Measurement results are respectively consistent with an ammonium persulfate oxidation volumetric method (JB / T9552.2-1999) and a xylenol orange spectrophotometric method (JB / T9552.5-1999). Relative standard deviations of ten parallel determinations are respectively 0.40% and 0.28%.
Owner:QINGDAO TIANHENG MACHINERY
Method for titration of zinc content in silver-copper-zinc alloy by ethylenediamine tetraacetic acid
InactiveCN103913457AReduce distractionsImprove accuracyMaterial analysis by observing effect on chemical indicatorEthylenediamineThiourea
The invention relates to a method for titration of the zinc content in a silver-copper-zinc alloy by ethylenediamine tetraacetic acid (EDTA). The method includes: weighing a unit sample in a beaker, dissolving the sample in nitric acid, taking part of the sample solution into a triangular flask, and adding a thiourea solution to mask the interference of silver copper; adding a bromocresol green indicator, a hexamethylene tetramine solution and water to adjust the pH to about 5.0, adding a xylenol orange indicator, and using an ethylenediamine tetraacetic acid (EDTA) standard solution to perform titration till the solution just changes from purple to grass green, recording the milliliter number of the consumed standard solution, and calculating the percent content results of each to-be-measured element according to a calculation formula. The method provided by the invention simplifies operation steps, shortens the testing time, reduces the interference of other elements on the sample, improves the accuracy of the test result, and is convenient for operation.
Owner:BEIJING INST OF NONFERROUS METALS & RARE EARTH
Method for directly determining aluminum content in aluminum-niobium alloy
InactiveCN105203698AReduce acidityImprove solubilityChemical analysis using titrationAluminum IonNiobium alloy
The invention relates to a method for directly determining aluminum content in aluminum-niobium alloy, and belongs to the technical field of chemical analysis and detection of hard alloy. The method comprises the following steps: dissolving a powdered aluminum-niobium alloy test sample in a sulfuric acid and nitric acid solution, adding an EDTA solution and distilled water to reduce the acidity of the solution, complexing aluminum ions and hydrolyzing niobium ions to facilitate the complete precipitation, obtaining a test solution of an Al-EDTA complex containing the niobium hydroxide precipitates, sufficiently releasing the aluminum ions, wherein the niobium hydroxide precipitates are too tiny to absorb the aluminum ions, so that the loss of the aluminum ions is reduced; and determining the aluminum ions in the mixed solution in a titration method, wherein xylenol orange in the titration method is used as an indication agent, NaF is used as a replacement agent, ZnCl2 is a standard solution for the titration, and titrating the replaced EDTA with the quantity equal to that of the aluminum. According to the method, the determination of the aluminum in the aluminum-niobium alloy is changed from an indirect difference method to a direct determination method, so that the detection accuracy is greatly improved, and a feasible method is provided for detecting the aluminum content in the niobium-aluminum alloy.
Owner:SHANGLUO TIANYE HIGH NEW MATERIAL CO LTD
Method determining content of nickel in nickel-titanium shape memory alloy
ActiveCN102928422AAccurate control of acidityAcidity controlMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationAlloyMethyl orange
The invention relates to a method for determining the content of nickel in a nickel-titanium shape memory alloy. The method comprises the following steps of: dissolving a sample with ammonium fluoride and nitric acid, and fluorating the sample; adding excessive EDTA (Ethylene Diamine Tetraacetic Acid) to make the EDTA completely complexed with nickel; regulating the acidity through an indicator; adding a buffer solution to control the acidity; titrating excessive EDTA by using a zinc scale; and determining the content of nickel by calculating. According to the method, the influence of titanium on the nickel during analysis is eliminated by masking the titanium, the end point mutation is obvious when the solution is titrated by regulating the acidity, and the more accurate content of nickel elements can be acquired by analyzing iron elements. The method for determining the content of the nickel in the nickel-titanium alloy has the advantages of simple process, obvious titration end point mutation, high recovery rate and high precision; the influence of Fe3<+> on the determination of the nickel can be eliminated; and the defects that methyl orange is used as an indicator to regulate the acidity, and xylenol orange is used as an indicator of complexometric titration end points in the conventional method to cause that the acidity control deviation is larger, and the titration end points are not obvious are overcome.
Owner:AECC AVIATION POWER CO LTD
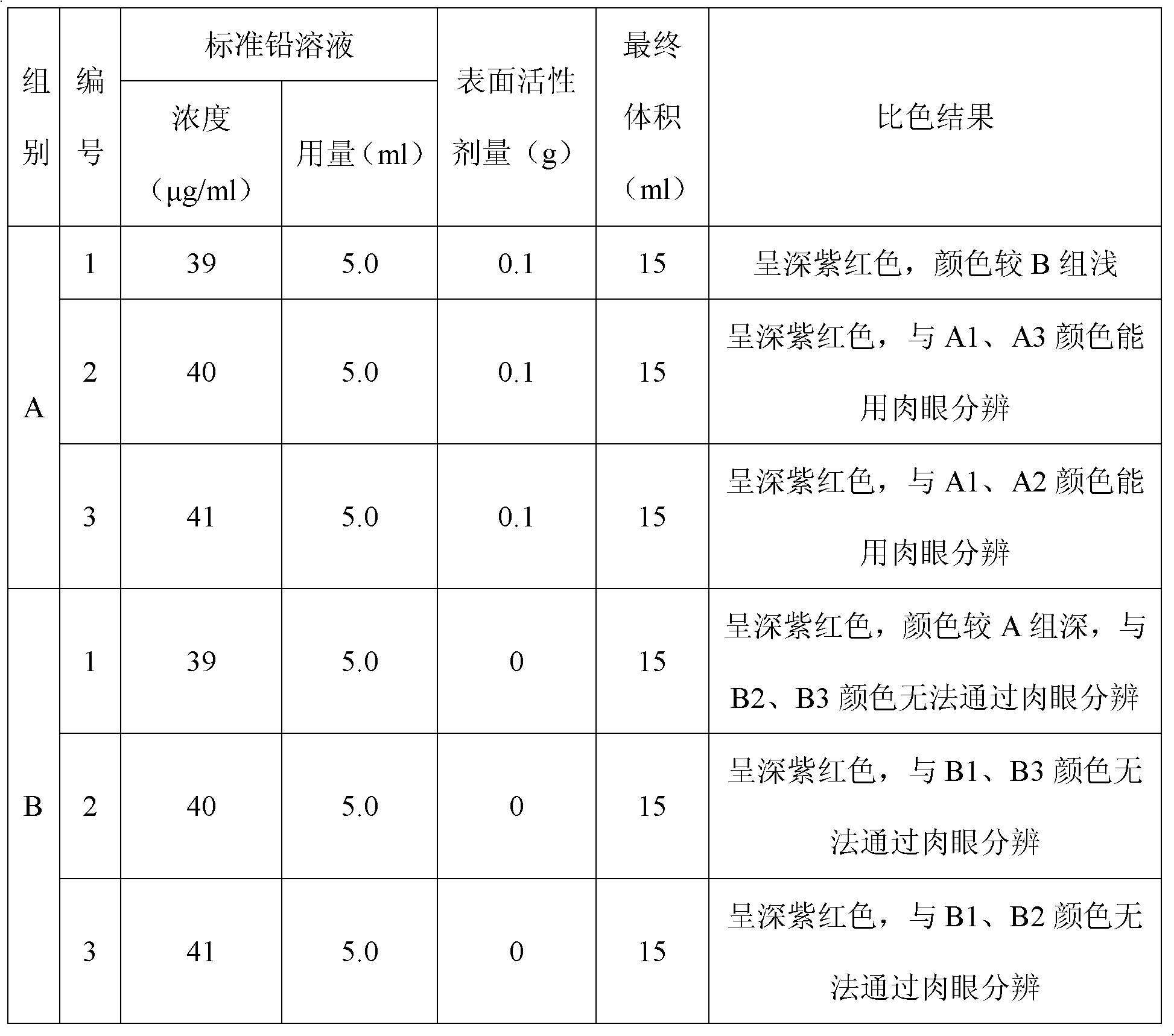
Front processing method of emulsion (grease) type cosmetic and quick detection method of lead in same
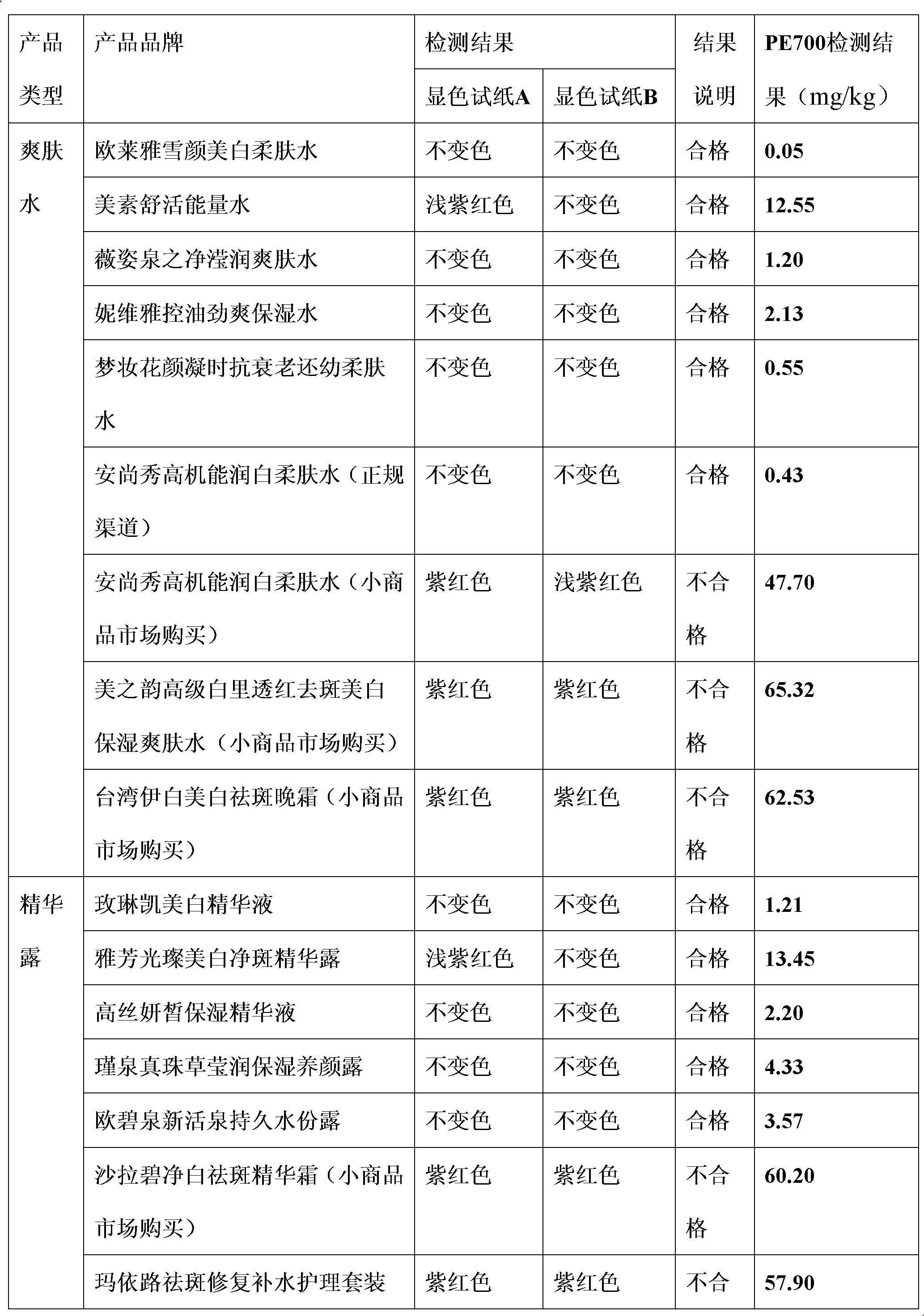
InactiveCN102589944AAvoid disturbing influenceSolve the effect of visual chromatic aberrationMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationEmulsionVisual observation
The invention discloses a front processing method of emulsion (grease) type cosmetic and a quick detection method of lead in the same. The front processing method includes adding emulsion type or grease type cosmetic into a container, then adding amphoterics and ethanol water with volume fraction ranging from 50% to 100%, and heating a colorimetric cylinder to obtain clear solution. The quick detection method of lead in the emulsion (grease) type cosmetic is that buffered solution containing 150-180mg / kg of xylenol orange is added into the clear solution obtained by the front processing under the heating condition to enable pH of a mixing system to be in a range from 4 to 6, clear sample solution to be tested is obtained after the mixture is evenly mixed, and then visual observation color comparison is conducted on the sample solution to be tested and standard sample solution obtained by standard lead solution through the same processing. The detection method of the lead is high in accuracy, good in repeatability and simple and quick to operate and has good using prospect.
Owner:CHINA JILIANG UNIV
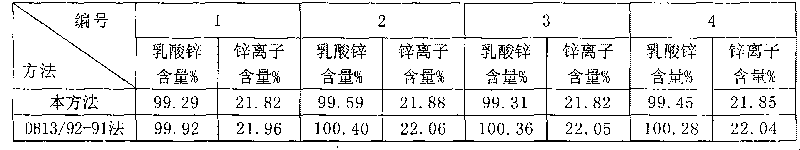
Method for measuring zinc lactate content by measuring zinc content
InactiveCN101696940AAvoid complexation reactionsObvious color changeMaterial analysis by observing effect on chemical indicatorSodium acetateThiourea
The invention discloses a method for measuring zinc lactate content by measuring zinc content, comprising the steps of carrying out a measurement experiment and a corresponding blank experiment on a zinc lactate test sample, and computing the zinc lactate content according to a volume difference of titration solution consumed in the two experiments and other data. The measurement experiment on the zinc lactate test sample comprises the following steps of taking the zinc lactate test sample, adding sulfuric acid solution till the zinc lactate test sample dissolves, adding water, ammonium fluoride solution, thiourea solution, ascorbic acid and uniformly mixing, adding acetic acid-sodium acetate buffer solution and xylenol orange indicating solution, titrating with ethylene diamine tetraacetic acid disodium standard titration solution till the color of the solution turning from red into bright yellow. By using the ammonium fluoride solution, the thiourea solution and the ascorbic acid to shelter metallic cations except zinc ions under the condition that the solution pH value is 5-6, the measurement of zinc ions has the advantages of higher degree of accuracy, simple and easy operation, and good reproducibility.
Owner:SICHUAN ANIMTECH FEED CO LTD
Method for detecting tin content in tin-lead solder by utilizing lead acetate chelatometric back-titration method
InactiveCN103185718AReduce distractionsImprove accuracyMaterial analysis by observing effect on chemical indicatorEthylene diaminePotassium nitrate
The invention discloses a method for detecting the tin content in tin-lead solder by utilizing a lead acetate chelatometric back-titration method. The method comprises the steps of acquiring parameters through the lead acetate chelatometric back-titration method by utilizing a prepared standard tin solution, and calculating the titer T of acetate lead to tin by utilizing a formula; acquiring parameters through the lead acetate chelatometric back-titration method by utilizing a prepared to-be-tested tin-lead solder sample solution; and finally calculating the tin content (Sn%) in the tin-lead solder through a formula. the specific method for acquiring parameters through the lead acetate chelatometric back-titration method comprises the steps of preparing a standard or to-be-tested sample solution for the tin-lead solder, taking the solution separately, adding a potassium nitrate solution and an EDTA ethylene diamine tetraacetic acid solution, heating and boiling, and cooling through running water; adding a hexamethylenetetramine solution, adding xylenol orange, titrating by using the standard acetate lead solution until the solution presents an orange red color, adding ammonium fluoride, laying aside, titrating again by using the standard acetate lead solution, recording the consumed milliliter number of the standard acetate lead solution during titration; and taking the parameters acquired in the method in an equation to obtain the final detection result.
Owner:BEIJING INST OF NONFERROUS METALS & RARE EARTH
Environment-friendly metal oil/rust-removing anti-rust solution
The invention discloses an environment-friendly metal oil / rust-removing anti-rust solution. The environment-friendly metal oil / rust-removing anti-rust solution is prepared from the following raw materials in parts by weight: 4-5 parts of benzoic acid, 3-5 parts of polypropylene, 1-2 parts of fatty alcohol-polyoxyethylene ether, 3-5 parts of cocamidopropyl betaine, 1-3 parts of coconut fatty acid diethanolamide, 2-3 parts of cetyl trimethyl ammonium bromide, 3-5 parts of tetraethylammonium bromide, 2-3 parts of tert-butyldiphenylsilane, 1-3 parts of di-o-tolyl-thiourea, 1-2 parts of ethylene diamine tetraacetic acid, 1-2 parts of xylenol orange, 2-3 parts of aluminum dihydrogen phosphate, 3-4 parts of sodium hexametaphosphate, 2-3 parts of acetic acid, 50-55 parts of phosphoric acid, 3-5 parts of sodium lignin sulfonate, 2-3 parts of disodium methylene dinaphthalenesulfonate and 50-70 parts of purified water. The environment-friendly metal oil / rust-removing anti-rust solution is multifunctional, and is not only applicable to line production to perform oil removal, rust removal, phosphatization, passivation and rust prevention on metal surfaces at one time, and is harmless to the human body and free from pollution to the environment.
Owner:司徒建辉
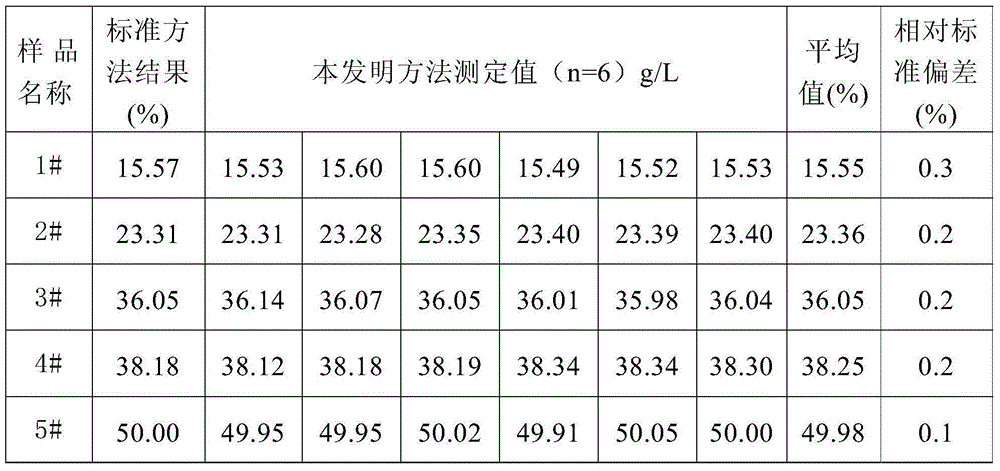
Method for quickly analyzing total iron in iron ore
InactiveCN104568942AObvious color jumpEasy to observeMaterial analysis by observing effect on chemical indicatorFerric hydroxideSulphosalicylic acid
The invention provides a method for quickly analyzing total iron in iron ore. The method comprises steps as follows: adding a mixed acid consisting of concentrated hydrochloric acid, concentrated nitric acid and concentrated sulfuric acid to an iron ore sample to decompose the sample, adding water for boiling until no sulfuric acid smoke exists, and cooling to the room temperature; dropwise adding an ammonia water solution until ferric hydroxide precipitates, then dropwise adding an excessive nitric acid solution until all ferric hydroxide precipitates are dissolved, then diluting the solution with water, and adjusting the pH value to range from 1 to 2; adding a sulphosalicylic acid solution, performing even mixing, dropwise adding an excessive EDTA (ethylene diamine tetraacetic acid) standard solution at the room temperature until the red color disappears, and recording the dosage of the EDTA standard solution; continuously adding a xylenol orange solution to the sample solution, dropwise adding a bismuth nitrate standard solution until the solution turns orange red from yellow, and recording the dosage of the bismuth nitrate standard solution. According to the method, the content of the total iron in the iron ore is determined by back titrating the excessive EDTA standard solution in the solution with the bismuth nitrate standard solution at the normal temperature, and the obtained result has high accuracy and precision.
Owner:GENERAL RESEARCH INSTITUTE FOR NONFERROUS METALS BEIJNG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com