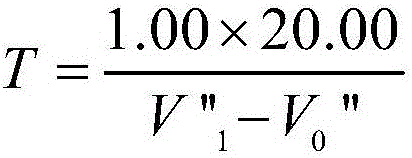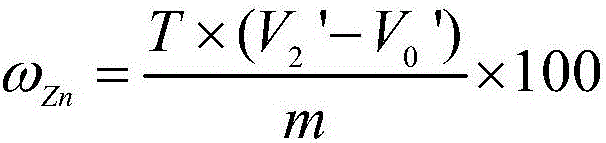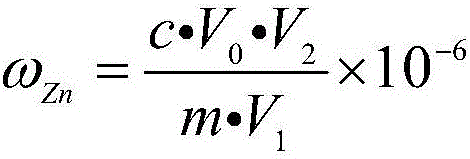Determination method of zinc in gold alloy
A method of determination, a technique for gold alloys, used in chemical analysis by titration, measuring devices, measurement of color/spectral properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Include the following steps:
[0056] (1) Weigh 0.5g sample, accurate to 0.0001g, place it in a 250mL beaker, add 8mL aqua regia, cover with a watch glass, heat at low temperature until the sample is completely dissolved, and evaporate to nearly dryness;
[0057](2) Add 4mL of hydrochloric acid, evaporate to near dryness, repeat 3 times, take it off, wash the watch glass and cup wall with water, add 1mL of sodium chloride solution, add water to 100mL, add dropwise 4mL of hydrazine hydrate solution under constant stirring, Heat and boil for 30 minutes to make the gold condense and precipitate, remove and cool to room temperature;
[0058] (3) Filter the test solution in a 400mL beaker with medium-speed quantitative filter paper, rinse the watch glass, beaker, filter paper and precipitation with water for 4 times, and the residue is used for recovery of gold;
[0059] (4) Place the filtrate on an electric stove and heat to evaporate to a volume of 15mL, add 4mL (1+1) sul...
Embodiment 2
[0067] Include the following steps:
[0068] (1) Weigh 0.8g sample, accurate to 0.0001g, place it in a 250mL beaker, add 10mL aqua regia, cover with a watch glass, heat at low temperature until the sample is completely dissolved, and evaporate to nearly dryness;
[0069] (2) Add 5mL of hydrochloric acid, then evaporate to nearly dryness, repeat 4 times, take it off, wash the watch glass and cup wall with water, add 1mL of sodium chloride solution, add water to 100mL, add dropwise 4mL of hydrazine hydrate solution under constant stirring, Heat and boil for 35 minutes to make the gold condense and precipitate, remove and cool to room temperature;
[0070] (3) Filter the test solution in a 400mL beaker with medium-speed quantitative filter paper, rinse the watch glass, beaker, filter paper and precipitation with water for 5 times, and the residue is used for recovery of gold;
[0071] (4) Place the filtrate on an electric stove and heat to evaporate to a volume of 20mL, add 5mL ...
Embodiment 3
[0079] Include the following steps:
[0080] (1) Weigh 1.0g sample, accurate to 0.0001g, put it in a 250mL beaker, add 12mL aqua regia, cover with a watch glass, heat at low temperature until the sample is completely dissolved, and evaporate to nearly dryness;
[0081] (2) Add 6mL of hydrochloric acid, then evaporate to near dryness, repeat 5 times, take it off, wash the watch glass and cup wall with water, add 1mL of sodium chloride solution, add water to 100mL, add dropwise 4mL of hydrazine hydrate solution under constant stirring, Heat and boil for 40 minutes to make the gold condense and precipitate, remove and cool to room temperature;
[0082] (3) Filter the test solution in a 400mL beaker with medium-speed quantitative filter paper, rinse the watch glass, beaker, filter paper and precipitation with water for 6 times, and the residue is used for recovery of gold;
[0083] (4) Place the filtrate on an electric stove and heat to evaporate to a volume of 25mL, add 6mL (1+1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com