Analysis method for quickly and accurately measuring zinc in zinc electrolyte
A zinc electrolyte and accurate measurement technology, applied in the detection field, can solve problems such as inaccurate and reliable analysis data, and achieve the effect of solving inaccurate and reliable analysis data
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2-7
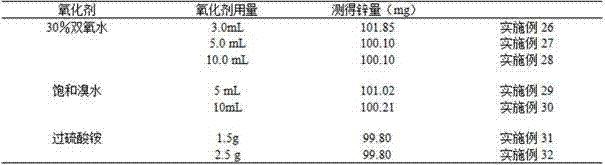
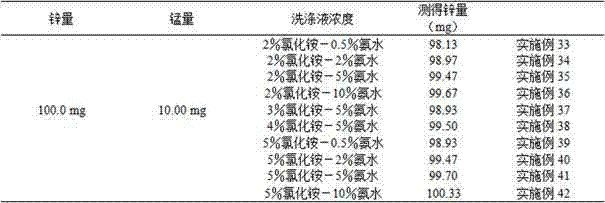
[0027] Pipette 6 parts of 1.00mL manganese-containing electrolyte into a 300mL triangular beaker, blow about 20mL of water, add 0.5, 1.0, 2.0, 3.0, 4.0, 5.0g of ammonium chloride, shake well, add 10mL of ammonia water, add 5.0mL For hydrogen peroxide, shake well, boil until no continuous bubbles are produced, destroy the hydrogen peroxide, put it in a 500mL triangular beaker with fast filter paper while it is hot, wash the beaker twice with hot 5% ammonium chloride-5% ammonia water washing solution, and precipitate 6-8 Second-rate. Shake the filtrate well, heat and concentrate to about 100 mL, remove and cool, add 0.1 g of ascorbic acid, shake well, add 3-4 drops of xylenol orange indicator, and adjust the solution with hydrochloric acid (1+1) and ammonia water (1+1). If it is yellow, add 5mL each of saturated sodium fluoride and thiourea, shake well, add 20mL acetic acid-sodium acetate buffer solution, shake well, and use EDTA standard titration solution until the solution ch...
Embodiment 8-12
[0032] Pipette 5 parts of 1.00mL manganese-containing electrolyte into a 300mL triangular beaker, blow about 20mL of water, add 3.0g of ammonium chloride, shake well, add 5.0, 8.0, 10.0, 12.0, 15.0mL of ammonia water respectively, add 5mL of hydrogen peroxide, Shake well, boil until no continuous bubbles are produced, destroy the hydrogen peroxide, put it in a 500mL triangular beaker with quick filter paper while it is hot, wash the beaker twice with hot 5% ammonium chloride-5% ammonia water washing solution, and precipitate 6-8 times. Shake the filtrate well, heat and concentrate to about 100 mL, remove and cool, add 0.1 g of ascorbic acid, shake well, add 3-4 drops of xylenol orange indicator, and adjust the solution with hydrochloric acid (1+1) and ammonia water (1+1). If it is yellow, add 5mL each of saturated sodium fluoride and thiourea, shake well, add 20mL acetic acid-sodium acetate buffer solution, shake well, and use EDTA standard titration solution until the solution...
Embodiment 13-19
[0037] Pipette 5 parts of 1.00mL manganese-containing electrolyte into a 300mL conical beaker, blow about 20mL of water, add 3.0g of ammonium chloride, shake well, add 10mL of ammonia water, add 1.0, 3.0, 4.0, 5.0, 6.0, 7.0, 9.0mL hydrogen peroxide, shake well, boil until no continuous bubbles are produced, destroy the hydrogen peroxide, put it in a 500mL triangular beaker with fast filter paper while it is hot, wash the beaker twice with hot 5% ammonium chloride-5% ammonia water washing solution, and precipitate 6 -8 times. Shake the filtrate well, heat and concentrate to about 100 mL, remove and cool, add 0.1 g of ascorbic acid, shake well, add 3-4 drops of xylenol orange indicator, and adjust the solution with hydrochloric acid (1+1) and ammonia water (1+1). If it is yellow, add 5mL each of saturated sodium fluoride and thiourea, shake well, add 20mL acetic acid-sodium acetate buffer solution, shake well, and use EDTA standard titration solution until the solution changes f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com