Quantum-dot marking test strip capable of quantitatively determining multiple indexes of blood infectious diseases and preparation method and quantitative determination method thereof
A quantitative detection and quantum dot technology, applied in measurement devices, instruments, scientific instruments, etc., can solve the problems of high consumption of reagents, complex detection process, long time required for analysis, etc., and achieve a wide range of applications.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: Quantum dot labeling test strip structure capable of quantitatively detecting multiple indicators of blood infectious diseases
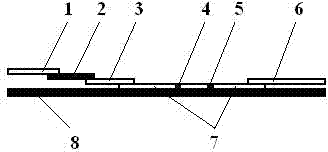
[0048] combine figure 1 be explained. figure 1 Among them, the quantum dot labeled test strip capable of quantitatively detecting multi-indicators of blood infectious diseases includes a sample pad 1, a red blood cell filter membrane 2, a marker pad 3, an analysis membrane 7, and Absorbent pad6.
[0049] The marking pad 3 of the test strip is a glass fiber membrane. The analysis membrane 7 is a nitrocellulose membrane, a nylon membrane, or a nitrocellulose / acetate mixed membrane, on which there are a detection zone (ie T zone) 4 and a quality control zone (ie C zone) 5 . Described backing 8 is polyester or plastic plate.
[0050] The marker pad 3 is coated with CdSe / ZnS QD 523 Labeled HBsAg monoclonal antibody (i.e. CdSe / ZnS QD 523 -HBsAg Mc), CdSe / ZnS QD 616 Labeled HIVAg (i.e. CdSe / ZnS QD 616 -HIVAg), CdSe / ZnS QDs 56...
Embodiment 2
[0051] Embodiment 2: Preparation method of quantum dot-labeled test strip capable of quantitatively detecting multi-indicators of blood infectious diseases
[0052] The preparation method of described test strip comprises the steps:
[0053] A. Antibody / antigen related to quantum dot coupling:
[0054] a) Take water-soluble CdSe / ZnS QD 523 、CdSe / ZnS QD 616 、CdSe / ZnS QD 568 、CdSe / ZnS QD 457 Use PBS buffer to adjust pH=6-9, respectively add EDC (l-(3-dimethylaminopropyl)-3-ethylcarbodiamine hydrochloride) and NHS (N-hydroxythiosuccinic acid imine) activated at room temperature for 10-60min;
[0055] b) Add HBsAg Mc, HIVAg, HCVAg, TPAg and vortex shake respectively for 0.5-3h;
[0056] c) Add bovine serum albumin (BSA) respectively, and block the reaction in the dark for 0.5-2h;
[0057] d) Each product was purified by centrifugation;
[0058] e) The precipitates of each product were resuspended and dispersed in PBS buffer to obtain CdSe / ZnS QD 523 -HBsAg Mc,...
Embodiment 3
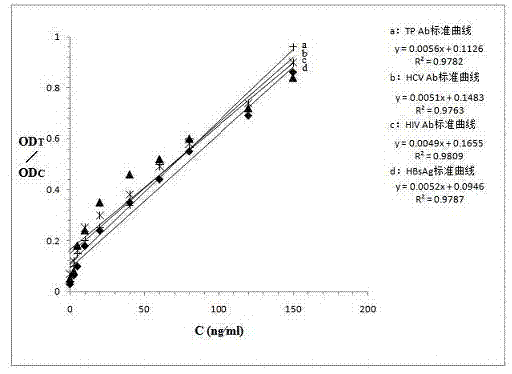
[0067] Example 3: Establishment of a standard curve for quantum dot-labeled test strips capable of quantitatively detecting multiple indicators of blood infectious diseases
[0068] (a) Using phosphate buffered solution (PBS) as the diluent, prepare 9 copies of HBsAg, HIVAb, HCVAb, and TPAb standard substances in series as shown in the table below.
[0069]
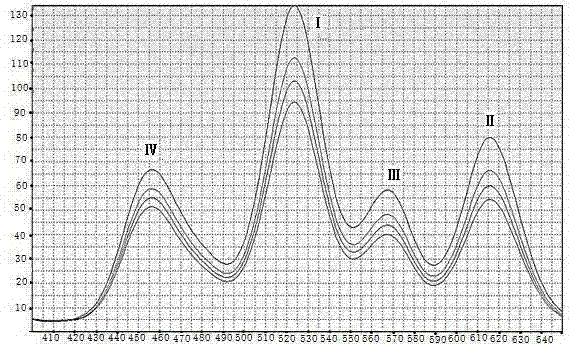
[0070] (b) The concentration of each standard substance in the table was detected by 10 quantum dot-labeled test strips under the same conditions with a detector for 10 times, and the T-band fluorescence intensity (OD t ) vs. C-band fluorescence intensity (OD c ), to get the mean and OD t / OD c ratio.
[0071] (c) Take the concentration of the standard series as the X-axis, and use OD t / OD c The ratio is used as the Y axis to obtain the standard curve corresponding to the fluorescence intensity and concentration. figure 2 .
[0072] The concentration of the standard series can also be used as the X-axis, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com