Application of nafamostat mesilate in preparation of neurological disease prevention and treatment medicines
A technology of nafamostat mesilate and medicine, which is applied in the application field of nafamostat mesilate in the preparation of drugs for preventing and treating nervous system diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
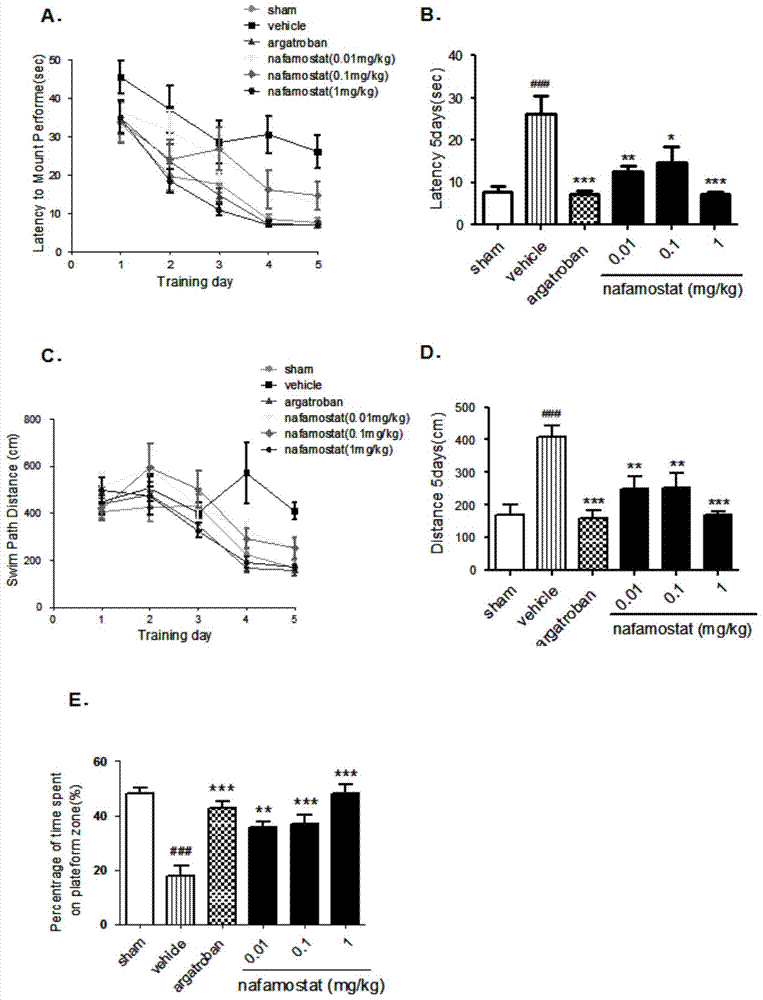
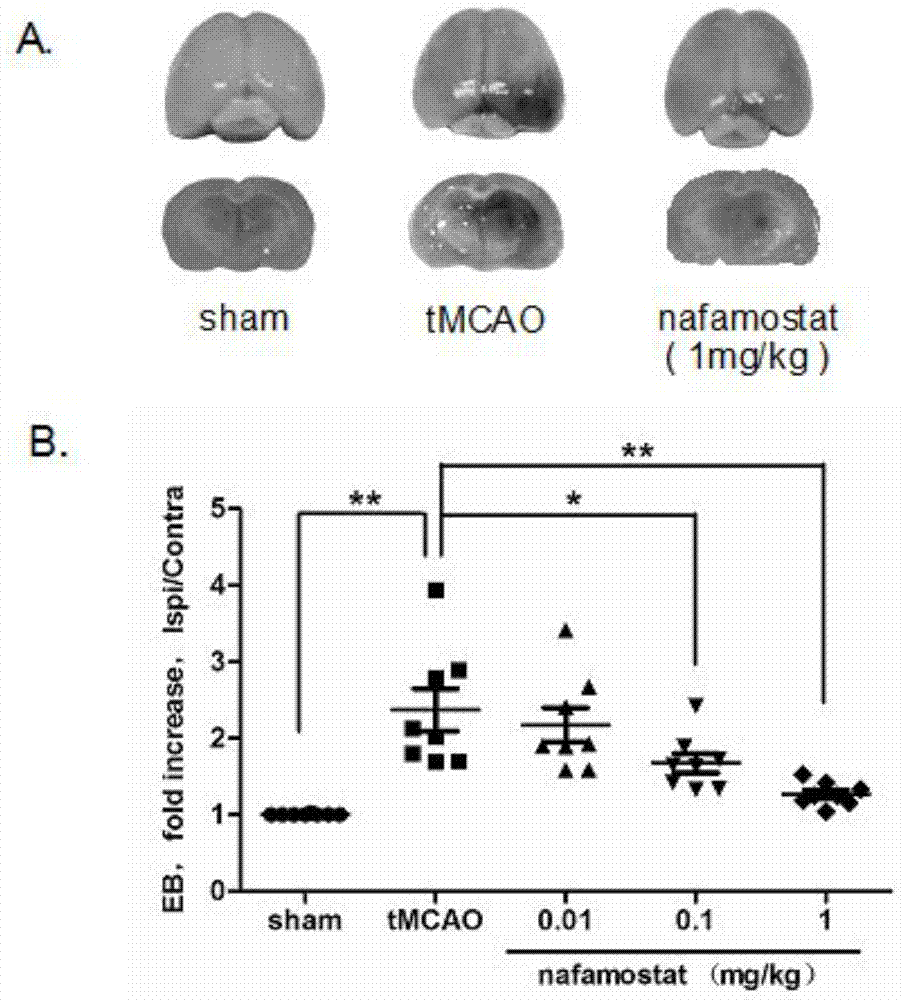
[0022] Study on inflammatory cell migration of nafamostat mesylate in rat brain injury
[0023] 1. Experimental method
[0024] 1) Animal grouping
[0025] A total of 85 clean-grade male SD rats (certificate number: SCXK (Zhejiang) 2008-0033), weighing 250-280g. Sham operation group: 10; tMCAO model group: 15; positive drug group: 15; low-dose nafamostat mesilate group: 15; middle-dose nafamostat mesilate group: 15; Nafamostat sulfonate high dose group: 15 rats.
[0026] 2) Establishment of rat brain injury model (tMCAO)
[0027] Referring to the method of Longa et al., the rat tMCAO model was prepared by the internal carotid artery suture method: the rat was anesthetized with 3% chloral hydrate (300mg / kg, ip), fixed in supine position, routinely disinfected, and the neck was cut in the middle The skin was bluntly dissected in all layers, exposing the right CCA. After the ICA and the ECA bifurcation of the external carotid artery were separated, the vagus nerve and trache...
Embodiment 2
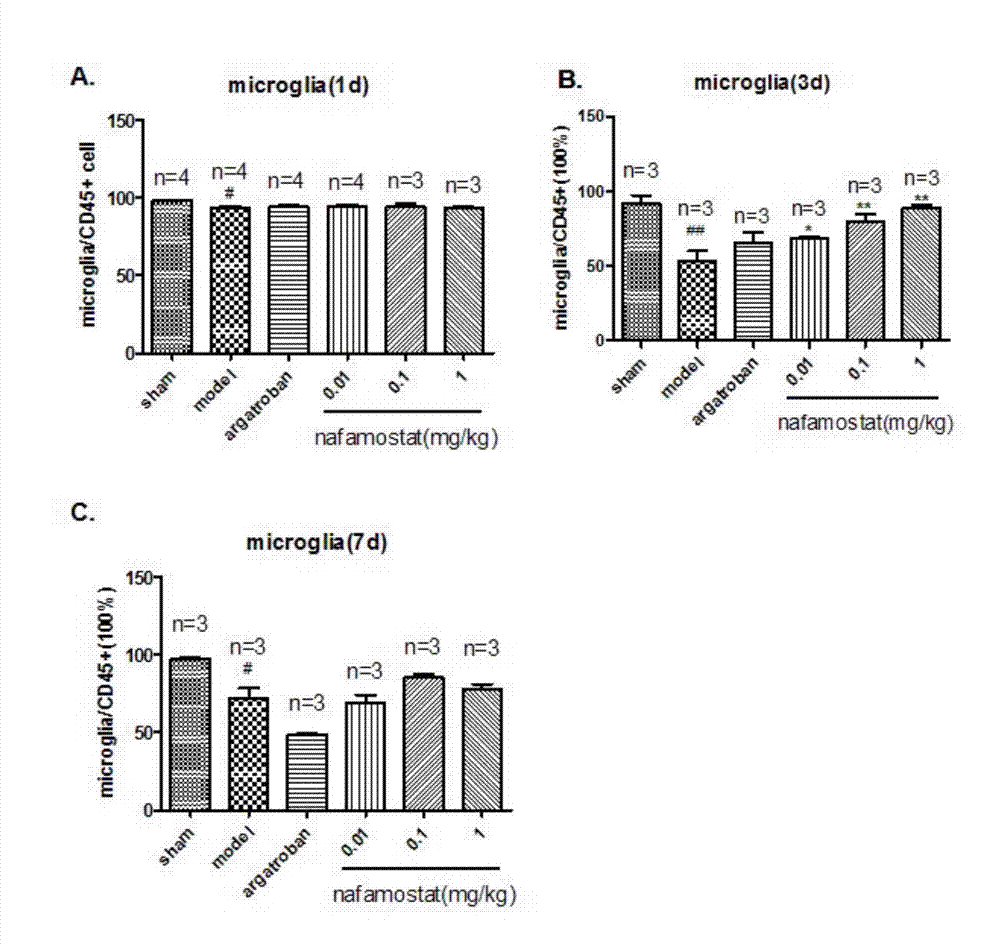
[0037] Study on the synthesis and release of inflammatory factors of nafamostat mesylate on brain injury in rats
[0038] 1. Experimental method
[0039] 1) Animal grouping
[0040] A total of 60 clean-grade male SD rats, weighing 250-280g. Sham operation group: 6; tMCAO model group: 12; positive drug group: 8; low-dose nafamostat mesilate group: 12; middle-dose nafamostat mesilate group: 12; Nafamostat sulfonate high-dose group: 12 rats.
[0041] 2) Establishment of rat brain injury model (tMCAO)
[0042] With embodiment 1.
[0043] 3) Method of administration
[0044] With embodiment 1.
[0045] 4) Index determination
[0046] After 22 hours of reperfusion, the rats were anesthetized with 3% chloral hydrate, and the heart was perfused with ice PBS. Immediately after taking the brain, it was frozen in liquid nitrogen.
[0047] ① Take an appropriate amount of cortical tissue from the brain lesion, extract mRNA, and perform qRT-PCR.
[0048] ②Take the cortical tissue ...
Embodiment 3
[0052] Effect of nafamostat mesylate on thrombin content and activity in rat brain after brain injury
[0053] 1. Experimental method
[0054] A total of 80 clean-grade male SD rats (certificate number: SCXK (Zhejiang) 2008-0033), weighing 250-280g. Sham operation group: 6; tMCAO model group: 15; positive drug group: 12; low-dose nafamostat mesilate group: 17; middle-dose nafamostat mesilate group: 15; Nafamostat sulfonate high dose group: 15 rats.
[0055] The model establishment and drug administration methods are the same as in Example 1. During the test, the animal is anesthetized, perfused with normal saline, and the brain is quickly taken out and placed on ice; sagittal slices are used to separate the left and right hemispheres; coronal slices of the damaged brain tissue are taken, 6-8 slices (The thickness of each slice is about 1 mm), and the striatum part of each slice is weighed separately. Transfer a part of the brain tissue into a doff tube, add 1 volume of PBS ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com