Synthesis of decitabine
A sitabine and reaction technology, which is applied in the field of drug synthesis, can solve the problem that the ratio of chlororibose azacytosine α configuration isomer is large, the content of sitabine α configuration impurities is high, and the β configuration chlororibose nitrogen The problem of low purity of heterocytosine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
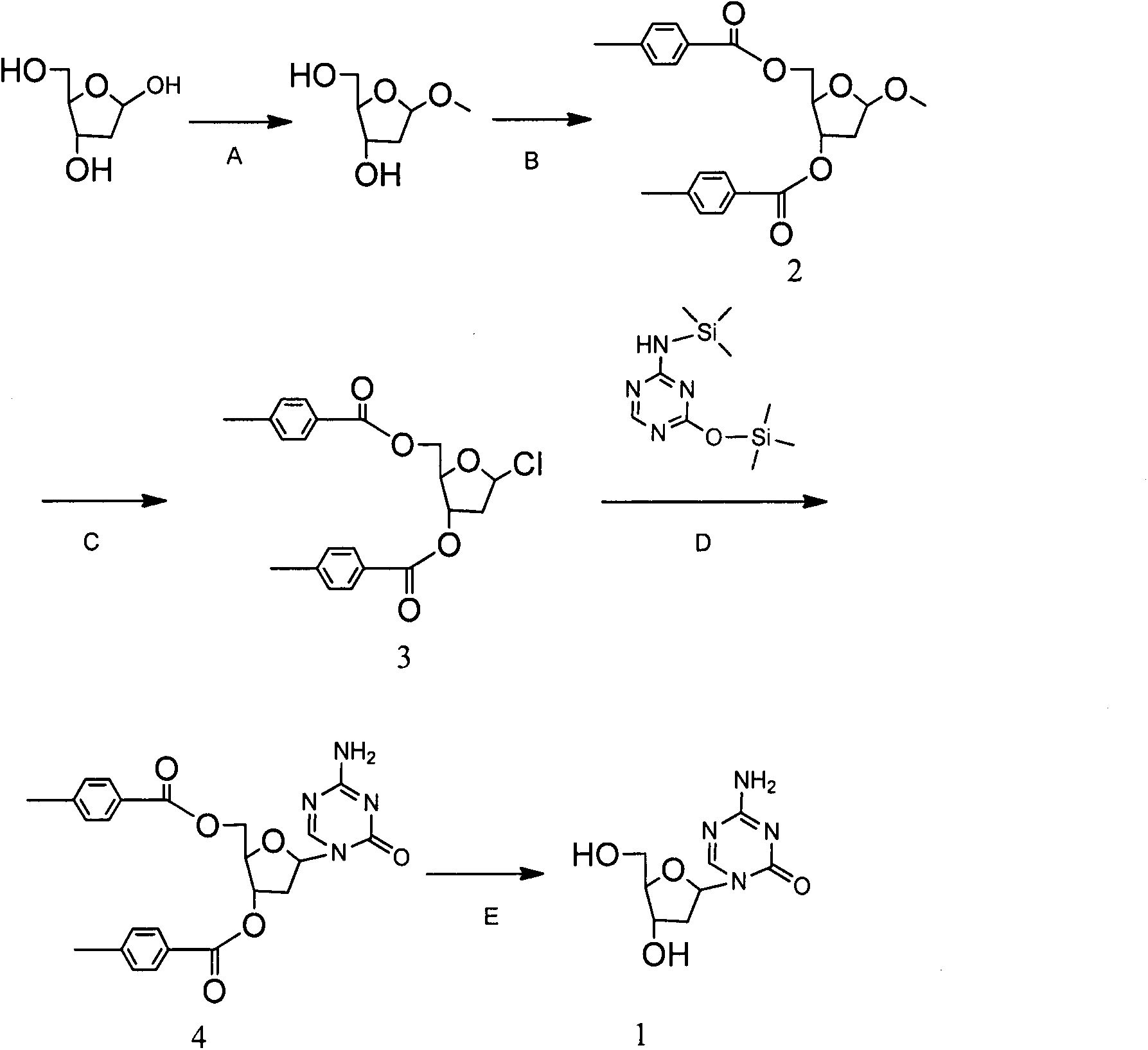
[0019] The preparation method of decitabine is made up of following five steps:
[0020] Step A: Synthesis of 1-methoxy-3,5-di-O-p-methylbenzoyl-2-deoxy-D-ribofuranose (2)
[0021] Add 15g (112mmol) 2-deoxy-D ribose, 15ml 1% HCl-CH 3 OH solution, 90ml of methanol, reacted at 30°C for 30min, added 0.375ml of pyridine and stirred for 5min, and evaporated the reaction solution to dryness under reduced pressure to obtain a viscous oil.
[0022] Step B: Dissolve 75ml of pyridine and transfer it to a four-necked reaction flask, add 33ml (247mmol) p-toluoyl chloride dropwise at -10°C, react at 25°C for 12h after dropping, add 60ml of dichloromethane, and slowly add it under stirring 90ml of 10% sodium carbonate solution, let stand to separate layers, extract the water phase with 30ml of dichloromethane, combine the dichloromethane phase, wash with 75ml of concentrated hydrochloric acid + 150g of crushed ice, 45ml of saturated brine, dry over anhydrous sodium sulfate, and filter with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com