Preparation method of halogen-free flame retardant nylon 66 polymer
A technology of flame retardant nylon and polymer, which is applied in the field of preparation of flame retardant nylon 66 to achieve the effect of high flame retardant decomposition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
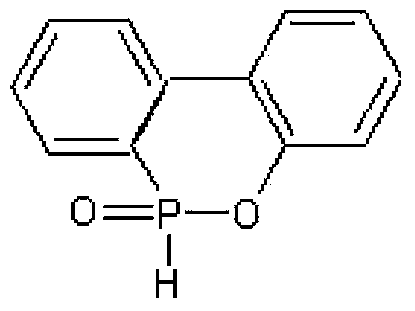
[0029] DDP (DOPO derivative I) is selected as the reactive flame retardant in this example. The DDP Chinese alias [(6-oxo-6H-dibenzo[c,e][1,2]oxaphosphine -6-yl)methyl]succinic acid, its molecular structure is shown in formula (e), physical and chemical properties: molecular weight: 346.27; appearance: white powder; effective content: ≥98.5%; melting point: 191-192°C; flash Point: 303.5°C; Boiling point: 578.3°C; Density: 1.48g / cm 3 .
[0030]
[0031] The process of this embodiment is as follows:
[0032] 75 grams of flame retardant DDP and 25 grams of hexamethylenediamine are neutralized in aqueous solution at room temperature to form a salt solution. 1.5 kg of nylon 66 salt and 50°C desalted water are prepared to form a 50% nylon 66 salt solution. The two salt solutions were added to a stainless steel high-temperature autoclave. After the "three pumping and three charging" of high-purity nitrogen and vacuum, the temperature and pressure are raised to 220 ° C and 20 k...
Embodiment 2
[0034] The selected flame retardant is the same as in Example 1. The process of this embodiment is: 75 grams of flame retardant DDP and 25 grams of hexamethylenediamine are neutralized in an aqueous solution at room temperature to form a salt solution. 1.5 kg of nylon 66 salt, 30 g of caprolactam and desalted water at 50°C were prepared to form a 50% nylon 66 salt solution. The two salt solutions were added to a stainless steel high-temperature autoclave. After the "three pumping and three charging" of high-purity nitrogen and vacuum, the temperature and pressure are raised to 220 ° C and 20 kg, and kept for 2 hours; then start to exhaust steam and slowly reduce the pressure, and after about 30 minutes, it drops to atmospheric pressure and starts to heat up at the same time to 275-285°C. Keep the temperature at 275-285°C, evacuate to -0.09MPa, and keep it for 30 minutes. Nitrogen is used to break the vacuum, and the material is discharged and pelletized. A flame-retardant n...
Embodiment 3
[0036] DOPO-MDA (DOPO derivative II) is selected as the reactive flame retardant in this example, and the molecular structure of DOPO-MDA is shown in formula (f). Its effective content is ≥98.5%; melting point: 250-253°C. Molecular Structure:
[0037]
[0038] The process of this embodiment is as follows:
[0039]90 grams of flame retardant DOPO-MDA and 31.5 grams of adipic acid are neutralized in aqueous solution at room temperature to form a salt solution. 1.5 kg of nylon 66 salt and 50°C desalted water are prepared to form a 50% nylon 66 salt solution. The two salt solutions were added to a stainless steel high-temperature autoclave. After the "three pumping and three charging" of high-purity nitrogen and vacuum, the temperature and pressure are raised to 220 ° C and 20 kg, and kept for 2 hours; then the steam is exhausted and the pressure is lowered slowly, and the pressure is lowered to atmospheric pressure after about 30 minutes, and the temperature starts to rise...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com