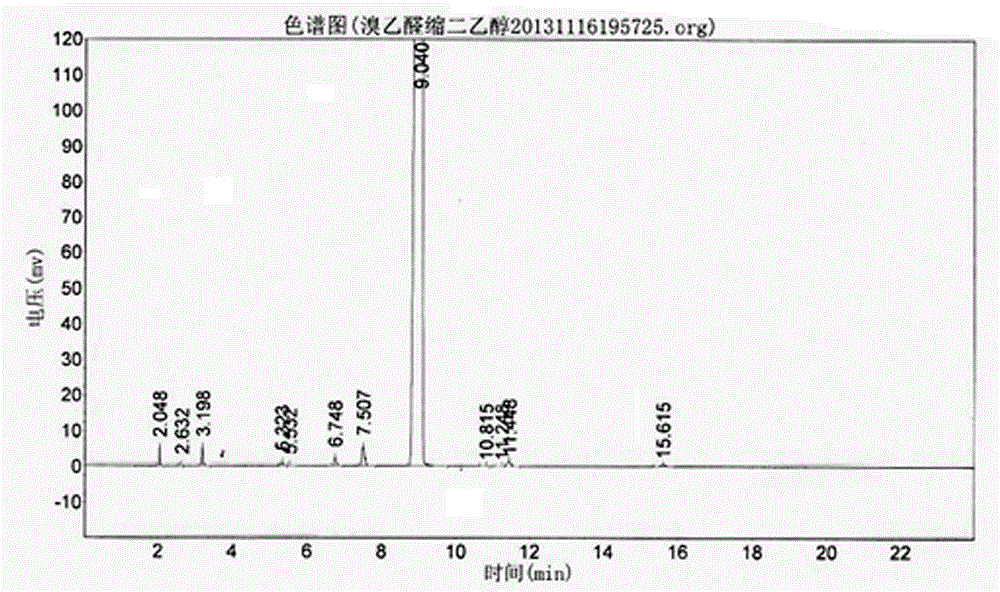
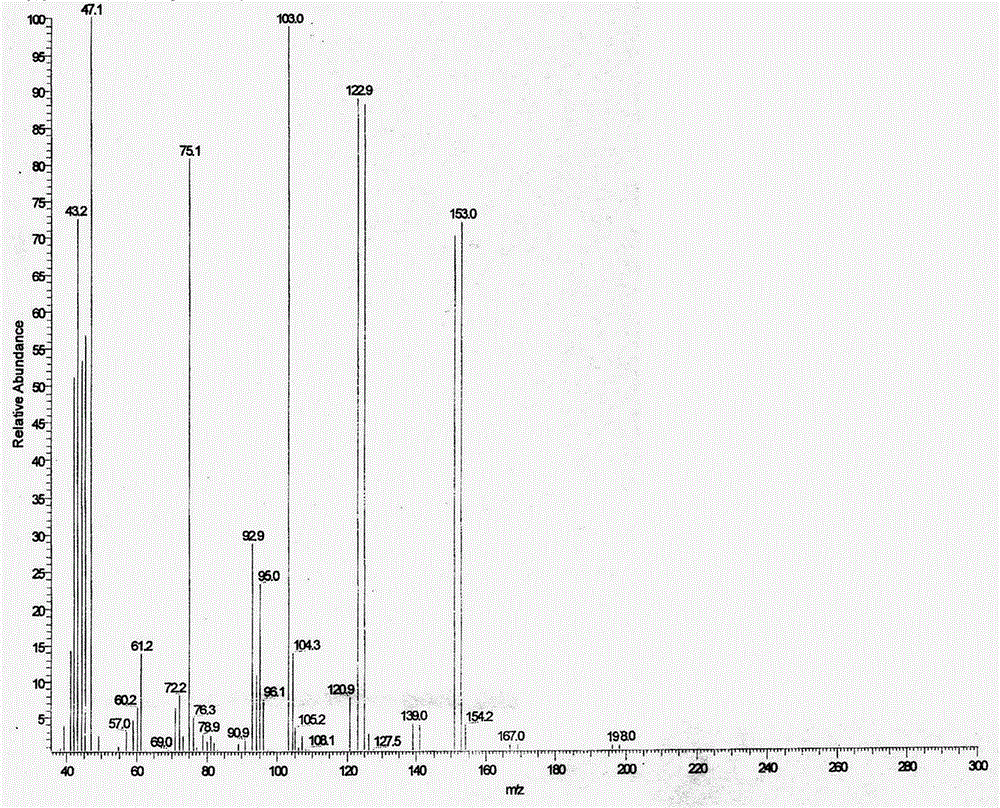
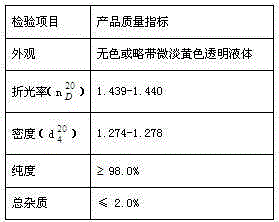
A kind of synthetic method of bromoacetaldehyde diethyl acetal
A technology of bromoacetaldehyde diethyl acetal and a synthesis method, which is applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problem of poor selectivity, difficult separation, and bromoacetaldehyde diethyl acetal products. Purity reduction and other problems, to achieve the effect of high purity and meet the requirements of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] ① Catalytic bromination reaction: Stir and dissolve the raw materials paraldehyde 132kg, copper bromide 1.58kg, concentrated sulfuric acid 0.53L and anhydrous ethanol 858L, cool to below -5°C with an ice-salt bath, and then slowly drop into the reaction kettle Add 475kg of elemental bromine, control the reaction temperature below 0°C, finish the dropwise addition in 3 hours, and react at -5~0°C for 1 hour to obtain about 1200kg of ethanol solution of bromoacetaldehyde, which is directly used in the next step of acetalization without separation reaction;
[0031] ②Acetalization reaction: Put 150kg of anhydrous sodium sulfate into the ethanol solution of bromoacetaldehyde obtained in the previous step, heat up to 35°C, keep warm for 5 hours, then add 300L of ice water, stir for 15 minutes at room temperature, and add 180kg of sodium carbonate Neutralize the reaction solution so that the pH of the reaction solution is 6~7, stir, let stand to separate layers, separate the o...
Embodiment 2
[0034] ① Catalytic bromination reaction: Stir and dissolve the raw materials paraldehyde 132kg, cuprous bromide 1.4kg, concentrated sulfuric acid 0.4L and 800L absolute ethanol, cool to -5°C with an ice-salt bath, and then slowly drop into the reaction kettle Add 460kg of elemental bromine, control the reaction temperature below 0°C, finish the dropwise addition in 3 hours, and react at -5~0°C for 1 hour to obtain about 1150kg of ethanol solution of bromoacetaldehyde, which is directly used in the next step of acetalization without separation reaction.
[0035] ②Acetalization reaction: Add 120kg of anhydrous magnesium sulfate to the ethanol solution of bromoacetaldehyde obtained in the previous step, heat up to 40°C, keep the temperature for 5 hours, then add 250L of ice water to the reaction solution, and stir for 15 minutes at room temperature. Add 150kg of sodium carbonate to neutralize the reaction solution, make the pH of the reaction solution 6~7, stir, let stand to sep...
Embodiment 3
[0038] ① Catalytic bromination reaction: Stir and dissolve the raw materials paraldehyde 132kg, copper powder 2.0kg, concentrated sulfuric acid 1.0L, and absolute ethanol 1050L, cool to below -5°C with an ice-salt bath, and then slowly drop into the reaction kettle Add 520kg of elemental bromine, control the reaction temperature below 0°C, complete the dropwise addition in 4h, and react at -5~0°C for 1.5h to obtain about 1500kg of ethanol solution of bromoacetaldehyde, which is directly used in the next step of acetal without separation Chemical reaction;
[0039] ②Acetalization reaction: Put 225kg of anhydrous sodium sulfate into the ethanol solution of bromoacetaldehyde obtained in the previous step, heat up to 40°C, keep warm for 6 hours, then add 450L of ice water, stir for 20 minutes at room temperature, and add 200kg of sodium carbonate Neutralize the reaction solution to make the pH of the reaction solution 6~7, stir, let stand to separate layers, separate the organic l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com