Human interleukin II mutant and application thereof
A technology of interleukin and mutants, applied in the fields of application, cytokines/lymphokines/interferons, animal/human proteins, etc., can solve problems such as restrictions, patient pain, and severe toxicity of interleukin II
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
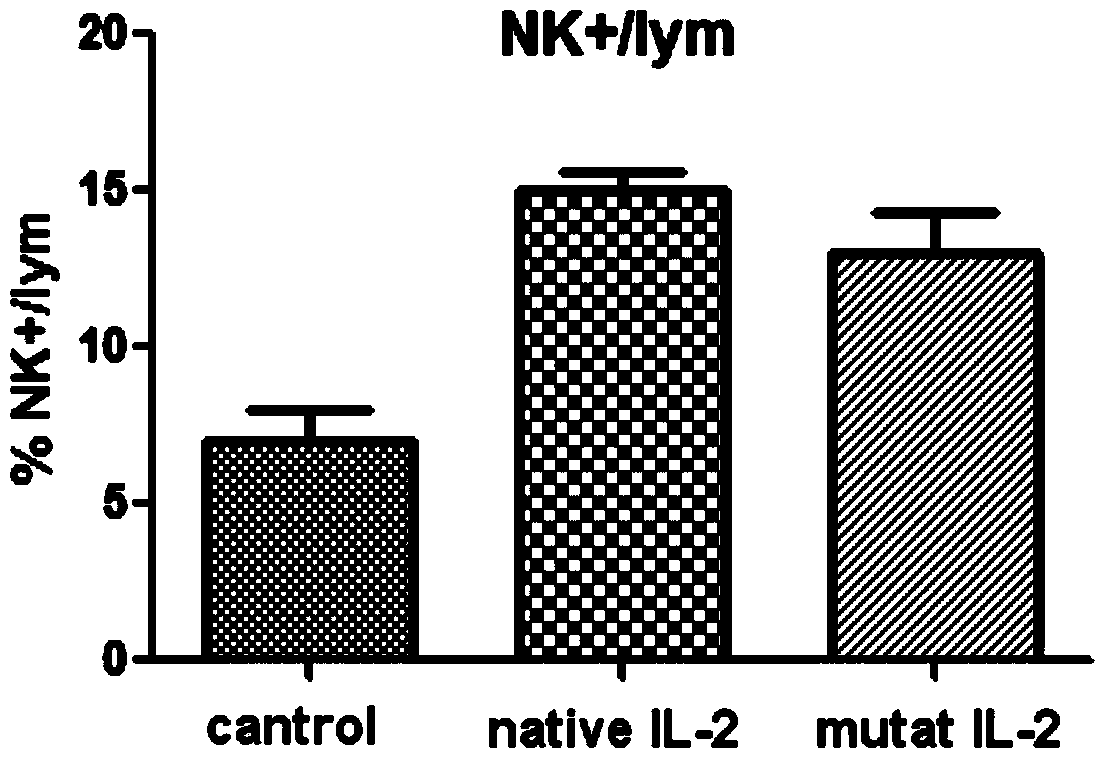
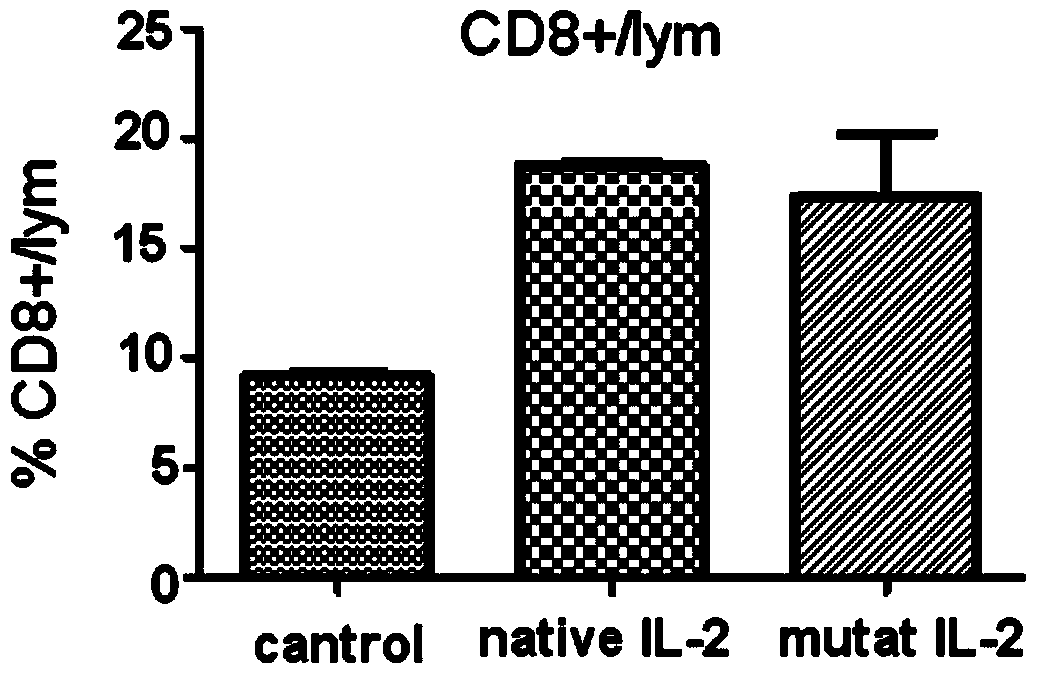
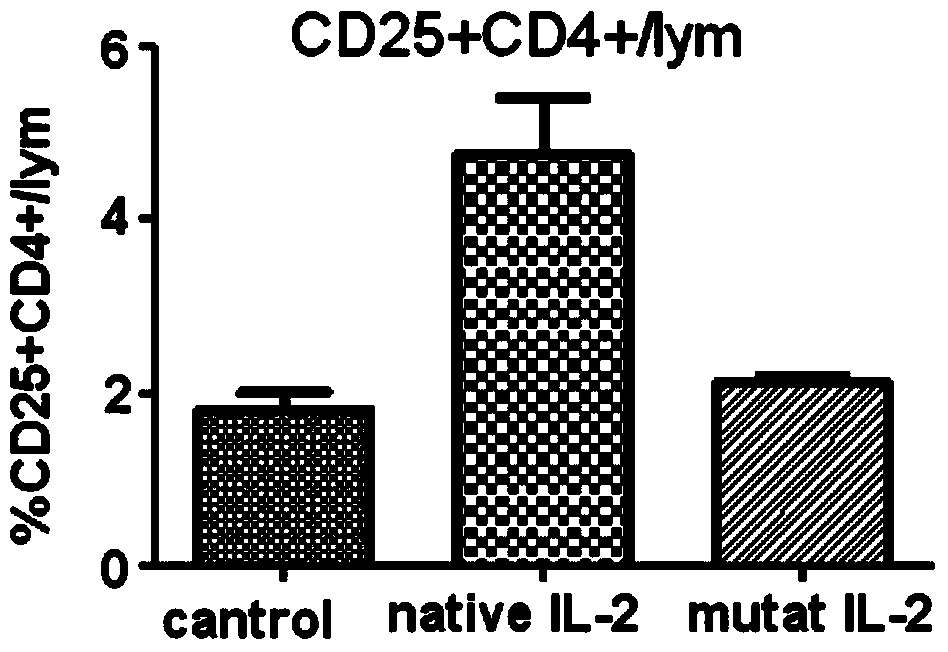
[0043] In one embodiment of the present invention, compared with SEQ ID No.2 (human interleukin II protoform) and SEQ ID No.3 (human interleukin II mutant of the present invention) in the sequence table of the present invention, the mutant is compared with the protoform , the mutation position and the replacement amino acid are as follows:
[0044] Table 1, the mutation site and replacement amino acid of the human interleukin II mutant of the present invention
[0045] site Prototype Amino Acid mutant amino acid 65 Pro Arg 80 Leu Phe 81 Arg Asp 85 Leu Val 86 Ile Val 92 Ile Phe
[0046] First, the gene sequence was designed according to the amino acid sequence of human interleukin II protozoa (NM_00586), and then the gene sequence was mutated. A mutant gene with an N-terminal polyhistidine tag was synthesized by the overlap extension method and further cloned into a multifunctional expression vector. The vector...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com