Kit for detecting infectious laryngotracheitis virus, newcastle disease virus and infectious bronchitis virus
A technology for chicken Newcastle disease virus and chicken infectivity, applied in the direction of microbe-based methods, biochemical equipment and methods, microbiological determination/testing, etc., can solve the problem of inability to explain specific and sensitive simultaneous detection, lack of chip specificity and sensitivity To achieve good application prospects, rapid detection, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 kit of the present invention
[0037] 1. Materials and instruments
[0038] The same experimental materials and instruments as mentioned above.
[0039] 2. Experimental method
[0040] 2.1 Preparation of PCR primers and detection probes
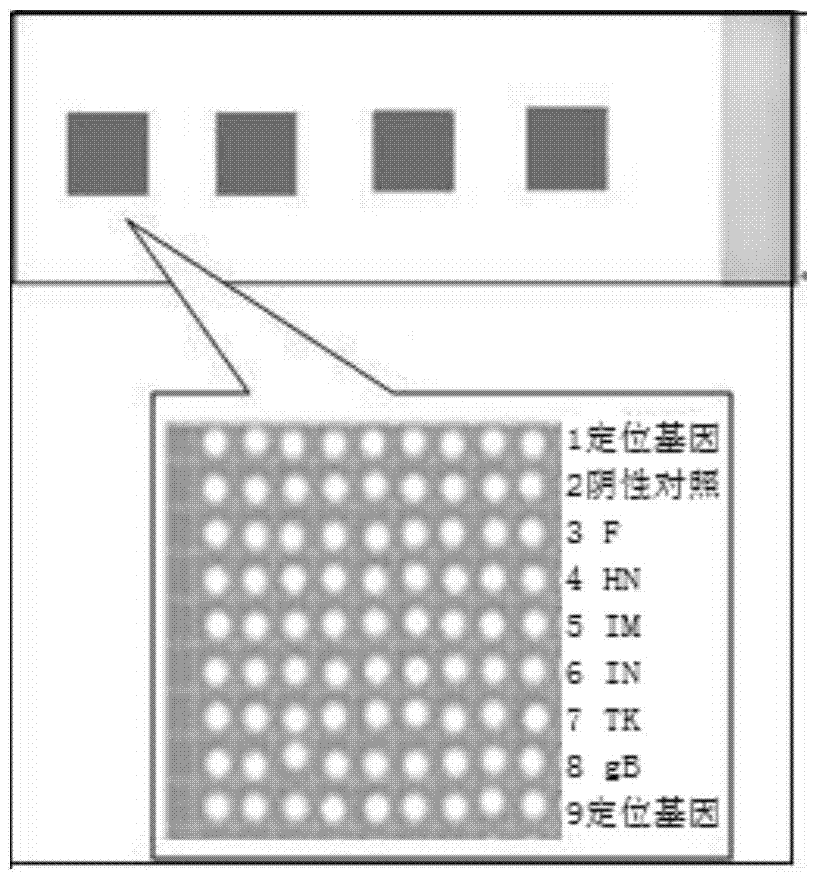
[0041] (1) Design of pathogen-specific probes: by comparing and analyzing the nucleic acid sequences of chicken Newcastle disease virus, chicken infectious bronchitis virus, and chicken infectious laryngotracheitis virus included in GenBnak, a conserved region sequence was selected: NDV-F / HN, IBV-IBM / IBN, ILTV-TK / gB. Design and detect multiple pairs of probes for conserved sequences, and select the probe sequences with strong specificity.
[0042] (2) Design of specific primers for probe sequences: specific primers were designed for the above conserved probe sequences using bioinformatics software DNAman, Primer5.0, etc. Specific primers were synthesized by Shanghai Bioengineering Company.
[0043...
Embodiment 2
[0209] Embodiment 2 detection method of the present invention
[0210] 1. Nucleic acid extraction
[0211] Extraction of viral RNA: The kit method is used to extract viral RNA, and the test uses a small amount of virus / liquid sample RNA extraction kit. Extract RNA according to the instructions of the kit, the method is as follows:
[0212] a) Take 300 μL of the sample to be tested and place it in a centrifuge tube, add 500 μL of RV solution, shake vigorously for 2 minutes, and then let stand at room temperature for 5 minutes.
[0213] b) Add 750 μL of isopropanol and shake gently.
[0214] c) Pipette 800 μL into the adsorption column and centrifuge at 12000 rpm for 30 s at 4°C.
[0215] d) Discard the liquid in the collection tube, transfer the remaining lysate into an adsorption column, and centrifuge at 12000r for 30s at 4°C.
[0216] e) Add 500 μL RP solution after discarding the collected solution, and centrifuge at 12000 rpm for 30 seconds at 4°C to remove protein.
...
Embodiment 3
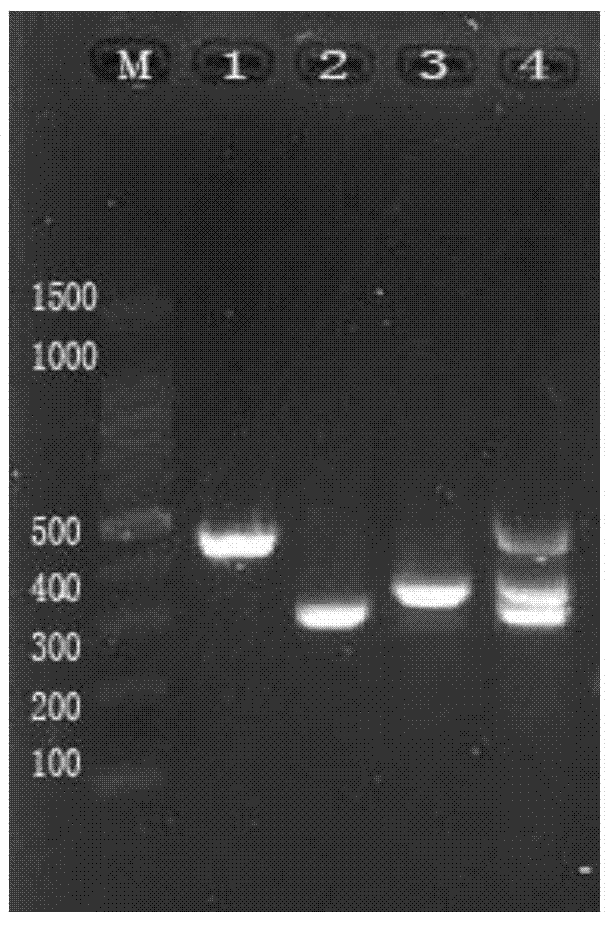
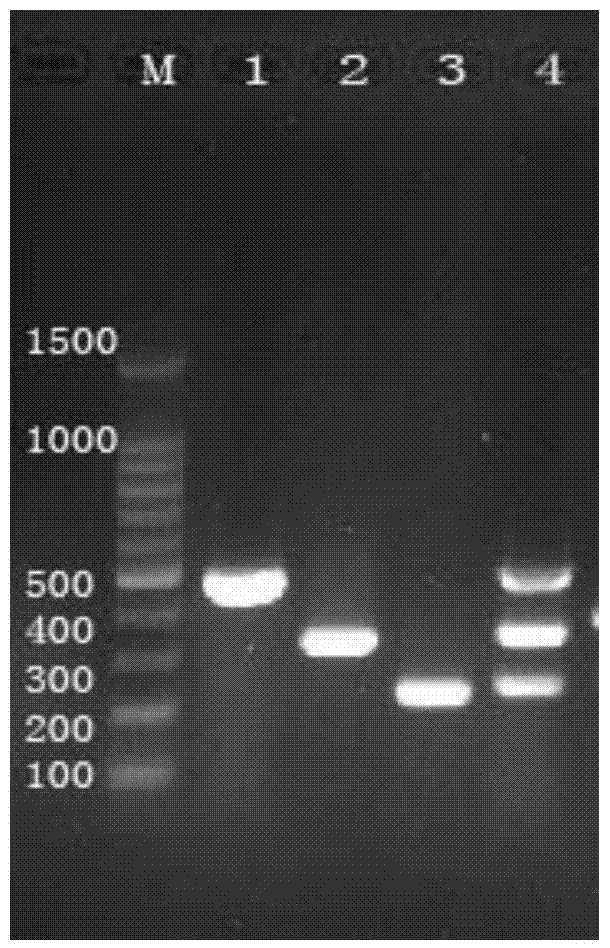
[0264] Embodiment 3 specificity test
[0265] 1. Test method
[0266] With the method of Example 2, five kinds of pathogens of NDV, IBV, ILTV, IBDV and AIV were detected to detect their specificity.
[0267] 2. Results
[0268] Experimental results such as Figure 4 As shown, the kit of the present invention can effectively detect NDV, IBV, and ILTV of the present invention, but will not detect other viruses, such as IBDV, AIV, indicating that the method of the present invention has strong specificity and will not amplify other viruses.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com