A kind of method for preparing isosorbide ethyl ether
A technology of isosorbide and ethoxylated isosorbide, which is applied in the field of preparing isosorbide ethyl ether, can solve the problems of many by-products, limiting the development and application of isosorbide etherified products, and low yield of etherified products, achieving corrosive mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
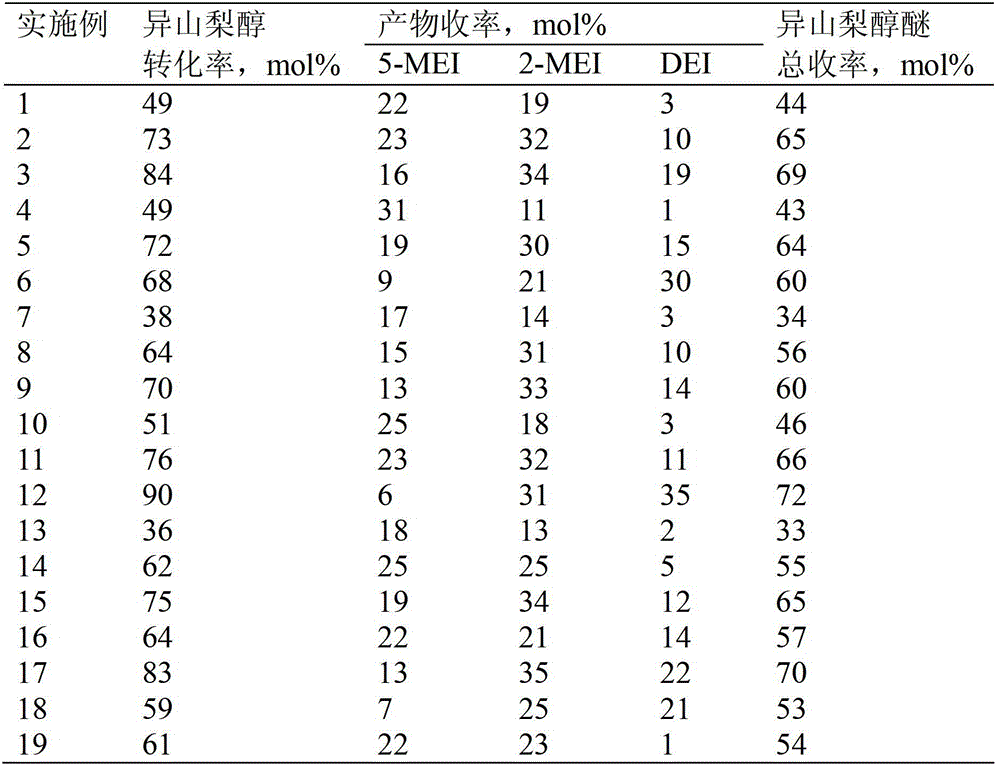
[0023]In a typical preparation, the etherification reaction of isosorbide and ethanol is carried out in a closed reactor. Put ethanol and isosorbide into a stainless steel reaction kettle according to a molar ratio of 50:1, add phosphotungstic heteropolyacid with 5% isosorbide mass, and use sulfolane as an etherification reaction solvent (5 times the mass of isosorbide ), filled with 3MPa nitrogen in advance, sealed the reactor, and reacted with magnetic stirring at 200°C for 2 hours. After the reactor was cooled to room temperature, the reaction solution was taken and analyzed by gas chromatography. The conversion rate of raw material isosorbide and the yield of etherification products were calculated by the internal standard method of gas chromatography. The obtained isosorbide etherification products include 5-ethoxy isosorbide (5-MEI), 2-ethoxy isosorbide (2-MEI) and isosorbide diethyl ether (DEI). Example 2
Embodiment 2
[0024] In a typical preparation, the etherification reaction of isosorbide and ethanol is carried out in a closed reactor. Put ethanol and isosorbide into a stainless steel reaction kettle according to a molar ratio of 50:1, add phosphotungstic heteropolyacid with 30% isosorbide mass, and use sulfolane as an etherification reaction solvent (5 times the mass of isosorbide ), filled with 3MPa nitrogen in advance, sealed the reactor, and reacted with magnetic stirring at 200°C for 2 hours. The conversion rate of raw material isosorbide and the yield of etherification products were calculated by gas chromatography internal standard method.
Embodiment 3
[0026] In a typical preparation, the etherification reaction of isosorbide and ethanol is carried out in a closed reactor. Put ethanol and isosorbide into the stainless steel reaction kettle according to the molar ratio of 50:1, add phosphotungstic heteropolyacid with 50% isosorbide quality, and use sulfolane as the etherification reaction solvent (5 times the isosorbide quality ), filled with 3MPa nitrogen in advance, sealed the reactor, and reacted with magnetic stirring at 200°C for 2 hours. The conversion rate of raw material isosorbide and the yield of etherification products were calculated by gas chromatography internal standard method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com