Process for preparing key intermediate of ivabradine
An intermediate and key technology, applied in the field of organic synthesis, can solve problems such as difficult reduction of cyano groups, difficult control of reactions, and complicated post-processing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
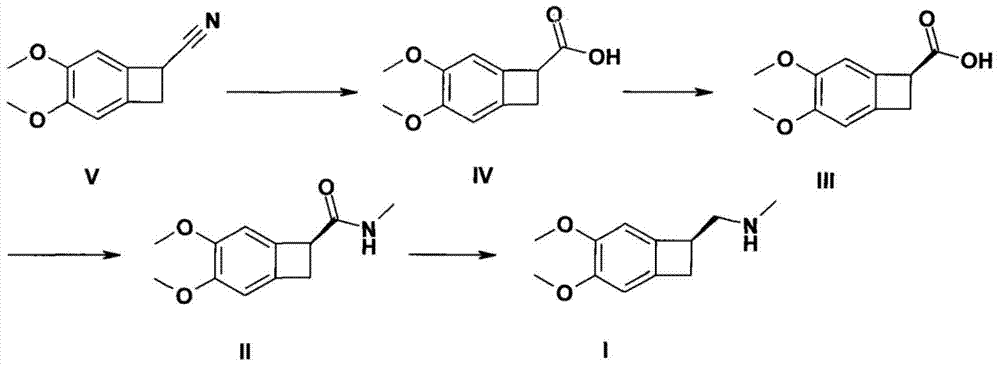
[0020] Under nitrogen protection, in a 500 ml four-neck round bottom flask, add 150 ml of pretreated anhydrous tetrahydrofuran, and add 0.1 mol of 4,5-dimethoxy-1-carbamoylbenzocyclocycline under stirring butane. In an ice-water bath, slowly add PdCl with stirring 2 / NaBH 4 (where NaBH 4 0.2mol, PdCl 2 1 mmol), after the dropwise addition was completed, the temperature was slowly raised to 50° C., and the reaction was carried out for 2 hours, and the reaction was monitored by TCL to complete. The reaction solvent tetrahydrofuran was distilled off in vacuo. Neutralize to neutral with 200 ml of saturated aqueous sodium bicarbonate solution, extract with 100 ml of dichloroethane, wash with saturated sodium chloride, and finally dry with anhydrous magnesium sulfate to obtain light yellow oil 1-(S)-4, 5-dimethoxy-1-methylaminomethylbenzocyclobutane, the yield is 90.2%, and the purity is above 98%.
Embodiment 2
[0022] Under nitrogen protection, in a 500 ml four-neck round bottom flask, add 150 ml of pretreated anhydrous tetrahydrofuran, and add 0.1 mol of 4,5-dimethoxy-1-carbamoylbenzocyclocycline under stirring butane. In an ice-water bath, slowly add PdCl with stirring 2 / NaBH 4 (where NaBH 4 0.3mol, PdCl 2 2 mmol), after the dropwise addition was completed, the temperature was slowly raised to 30° C., and the reaction was carried out for 3 hours, and the reaction was monitored by TCL to complete. The reaction solvent tetrahydrofuran was distilled off in vacuo. Neutralize to neutral with 200 ml of saturated aqueous sodium bicarbonate solution, extract with 100 ml of dichloroethane, wash with saturated sodium chloride, and finally dry with anhydrous sodium sulfate to obtain light yellow oil 1-(S)-4, 5-Dimethoxy-1-methylaminomethylbenzocyclobutane, the yield is 95.8%, and the purity is above 98%.
Embodiment 3
[0024] Under nitrogen protection, in a 500 ml four-neck round bottom flask, add 150 ml of pretreated anhydrous tetrahydrofuran, and add 0.1 mol of 4,5-dimethoxy-1-carbamoylbenzocyclocycline under stirring butane. In an ice-water bath, slowly add PdCl with stirring 2 / NaBH 4 (where NaBH 4 0.1mol, PdCl 2 1 mmol), after the dropwise addition was completed, the temperature was slowly raised to 40° C., and the reaction was carried out for 3 hours, and the reaction was completed by TCL monitoring. The reaction solvent tetrahydrofuran was distilled off in vacuo. Neutralize to neutral with 200 ml of saturated aqueous sodium bicarbonate solution, extract with 100 ml of dichloroethane, wash with saturated sodium chloride, and finally dry with anhydrous magnesium sulfate to obtain light yellow oil 1-(S)-4, 5-dimethoxy-1-methylaminomethylbenzocyclobutane, the yield is 92.7%, and the purity is above 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com