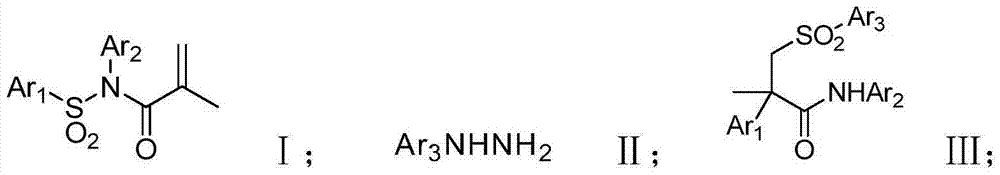Method for synthesizing alpha-aryl-beta-sulfonyl amide
A technology for sulfonyl amide and sulfonyl acrylamide, which is applied in the field of synthesizing α-aryl-β-sulfonyl amide, can solve the problems of high reaction cost, many reaction steps, complicated production process and the like, and achieves convenient post-processing, Effects that are easy to operate and simple to synthesize
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
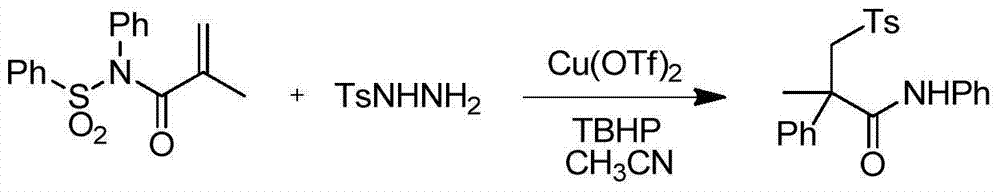
Embodiment 1
[0027] The molar ratio of N-phenylsulfonylacrylamide to p-toluenesulfonylhydrazide is 1:1.2, acetonitrile is used as solvent, and the amount of oxidant tert-butyl hydroperoxide is N-phenyl N-sulfonylacrylamide The molar ratio is 2 times.
[0028] Add 4.7g of N-phenylsulfonyl hydrazide acrylamide and 3.4g of p-toluenesulfonyl hydrazide to the round bottom flask, add 1.1g of copper trifluoromethanesulfonate, dissolve with acetonitrile and add the oxidant tert-butyl hydroperoxide 3.9 g, the above solution was reacted at 80°C for 3 hours.
[0029] Concrete reaction formula is as follows:
[0030]
[0031] After the reaction was completed, the product was separated and purified by column chromatography to obtain the corresponding α-phenyl-β-p-toluenesulfonylamide. The corresponding melting point was 177°C and the yield was 87%.
[0032] 1 H NMR (500MHz, CDCl 3 )δ7.52(d, J=8.3Hz, 2H), 7.31(d, J=7.5Hz, 2H), 7.26(t, J=8.1Hz, 3H), 7.17(dd, J=13.4, 8.2Hz, 4H), 7.07(dd, J=15.4, ...
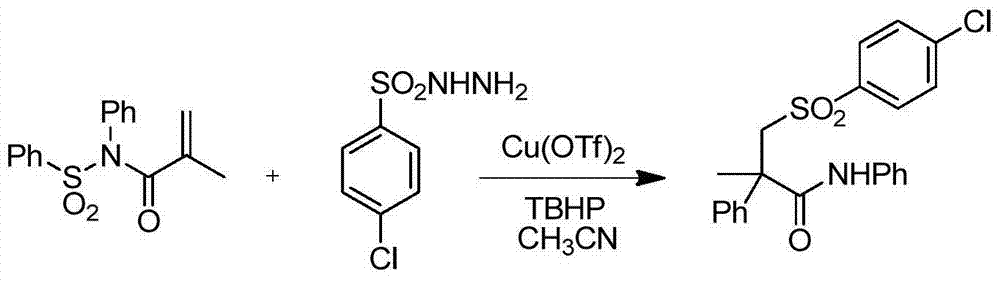
Embodiment 2
[0034] The molar ratio of N-phenylsulfonyl hydrazide acrylamide to p-toluenesulfonyl hydrazide is 1:1.5, add raw materials into the flask and add 4.7g of N-phenylsulfonyl hydrazide acrylamide, p-toluenesulfonyl Hydrazine 4.2g, add copper trifluoromethanesulfonate 1.1g, after dissolving with acetonitrile, add oxidant tert-butyl hydroperoxide 3.9g, other operation is the same as embodiment 1.
[0035] After the reaction was completed, the product was separated and purified by column chromatography to obtain the corresponding α-phenyl-β-p-toluenesulfonylamide. The corresponding melting point was 177° and the yield was 84%.
Embodiment 3
[0037] The molar ratio of N-phenyl N-sulfonyl hydrazide acrylamide to p-toluenesulfonyl hydrazide is 1:2, add raw materials into the flask and add 4.7g of N-phenyl N-sulfonyl hydrazide acrylamide, p-toluene sulfonyl hydrazide Benzenesulfonyl hydrazide 5.6g, add copper trifluoromethanesulfonate 1.1g, add oxidant tert-butyl hydroperoxide 3.9g after dissolving with acetonitrile, other operations are the same as embodiment 1
[0038] After the reaction was completed, the product was separated and purified by column chromatography to obtain the corresponding α-phenyl-β-p-toluenesulfonylamide. The corresponding melting point was 177°, and the yield was 80%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com