Preparation method of isoxaflutole
A technology of isoxaflutole and a compound is applied in the field of preparation of isoxaflutole-5-cyclopropylisoxazole), can solve the problems such as high cost of isoxaflutole, and achieves low cost of raw materials, The effect of thorough response and short response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
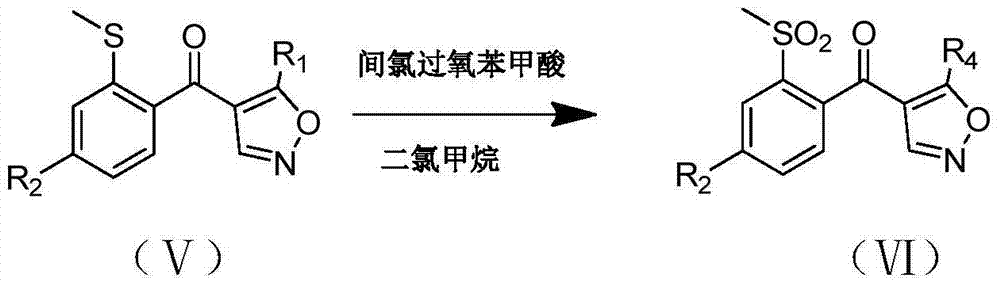
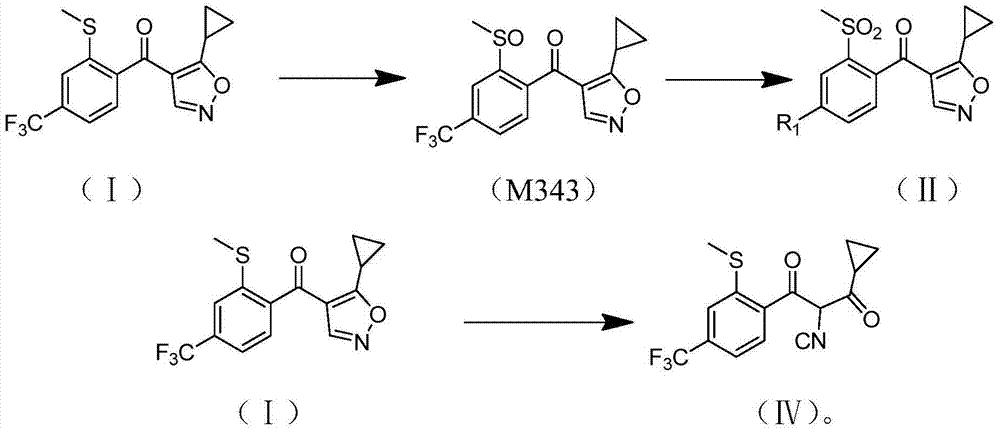
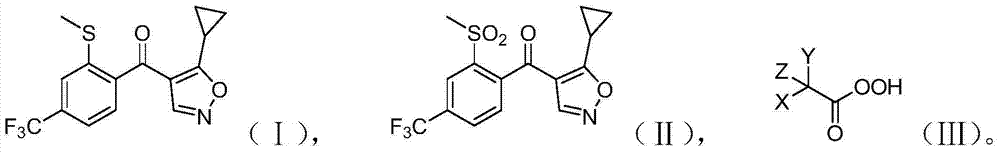
[0020] The present invention provides a preparation method of isoxaflutole, the structure of said isoxaflutole is shown in formula (II), the method comprises contacting the compound shown in formula (I) with an oxidizing agent, said The oxidant contains a peroxy organic acid; the peroxy organic acid is a compound shown in formula (III), and in formula (III), X, Y and Z are the same or different, each independently being hydrogen, halogen, C1 -one of C3 substituted or unsubstituted hydrocarbon groups,
[0021]
[0022] The preparation method of the above-mentioned isoxaflutole provided by the present invention can ensure that the reaction is carried out at a lower temperature and can ensure that there is no or only a small amount of by-products; and the above-mentioned specific peroxyorganic acid of the present invention can be used in the reaction The above-mentioned preparation method of the present invention also has the advantages of short reaction time, thorough reactio...
Embodiment approach
[0054] According to a preferred embodiment of the present invention, the preparation method of the isoxaflutole comprises supplying the compound represented by formula (I) with the organic acid corresponding to the compound represented by formula (III) and hydrogen peroxide physical contact.
[0055] In the method of the present invention, after the reaction is completed, a small amount of reducing agent can also be added to the reaction system to remove the unreacted oxidizing agent. The present invention has no special limitation on the type of the reducing agent, which can be Various reducing agents commonly used in the field, for example, sodium sulfite and / or sulfur dioxide are preferably used in the present invention.
[0056] The degree of progress of the reaction in the present invention can be detected by various methods routinely used in the art, for example, in the present invention, high performance liquid chromatography (HPLC) is preferably used for detection.
...
Embodiment 1
[0064] Add 32.7 g (0.1 mol) of 4-(4-trifluoromethyl-2-methylthiobenzoyl)-5-cyclopropylisoxazole and 150 g of dichloroacetic acid into a 500 ml reaction flask, and stir to dissolve. Then add 30% by weight of hydrogen peroxide (H 2 o 2 ) was 45.3g (0.40mol) in total, and was incubated at 25°C for 3h, and the conversion rate of raw materials was found to be 100% by HPLC sampling. Then add a small amount of sodium sulfite to remove excess oxidant in the resulting mixture, add 200g of dichloromethane to the concentrate after removing the solvent under reduced pressure, and wash 2 times with deionized water, each 50g, then remove the dichloromethane under reduced pressure. Chloromethane, recrystallized from ethanol, and filtered.
[0065] The filtered solid was dried under an infrared lamp to obtain 35.5 g of a white solid. The purity of the product detected by HPLC was 97.5%, and the yield was 96.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com