3,4-open-ring helianthemum diterpenoid type compounds, preparing method thereof and applications of the compounds
A compound and diterpenoid technology, applied in the field of 3,4-split-helicanane-type diterpenoids, can solve problems such as unclear active ingredients, achieve anti-inflammatory activity, high clinical application value and development prospects , less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Extraction and identification of compounds I and II of the present invention (method 1)
[0036] After pulverizing the leaves (12.5kg) of Auranthus nudiflora, extracting them under reflux with water for 3-10 times, the ratio of the amount of solvent added to the mass of Auranthus nudiflora leaves is (8-15)L:1kg; The time is 3 hours, the solvent is recovered under reduced pressure, the extract is concentrated, the extracts are combined, filtered, evaporated to dryness under reduced pressure, and the extract is obtained. The extract was suspended in 8000ml of distilled water and extracted with chloroform (3 times). The obtained extracts were combined and concentrated to obtain about (50 g) of chloroform extract. a. Take the chloroform extract and load it onto a silica gel chromatographic column. Different gradients of petroleum ether-acetone mixed solvents (petroleum ether:acetone—200:1-0:100, v / v) were used for elution, and fractions were collected according ...
Embodiment 2
[0038] Example 2 Extraction and identification of compounds I and II of the present invention (method 2)
[0039] After pulverizing the leaves (12.5kg) of Auranthus nudiflora, extracting them under reflux with water for 3-10 times, the ratio of the amount of solvent added to the mass of Auranthus nudiflora leaves is (8-15)L:1kg; The time is 3 hours, the solvent is recovered under reduced pressure, the extract is combined and concentrated to 8000ml, separated by HPD100 macroporous resin adsorption chromatographic column, and then washed with H 2 O, volume fraction 30%, volume fraction 60%, volume fraction 90% ethanol solution elution; Ether: acetone—200:1-0:100, v / v) for elution, and fractions were collected according to the different gradients of the eluent used. b. The (petroleum ether: acetone—100:12, v / v) fractions were separated by column chromatography packed with thin-layer chromatography silica gel. Different gradients of petroleum ether-dichloromethane mixed solvents...
Embodiment 3
[0041] Embodiment 3 Characterization of compounds I and II of the present invention
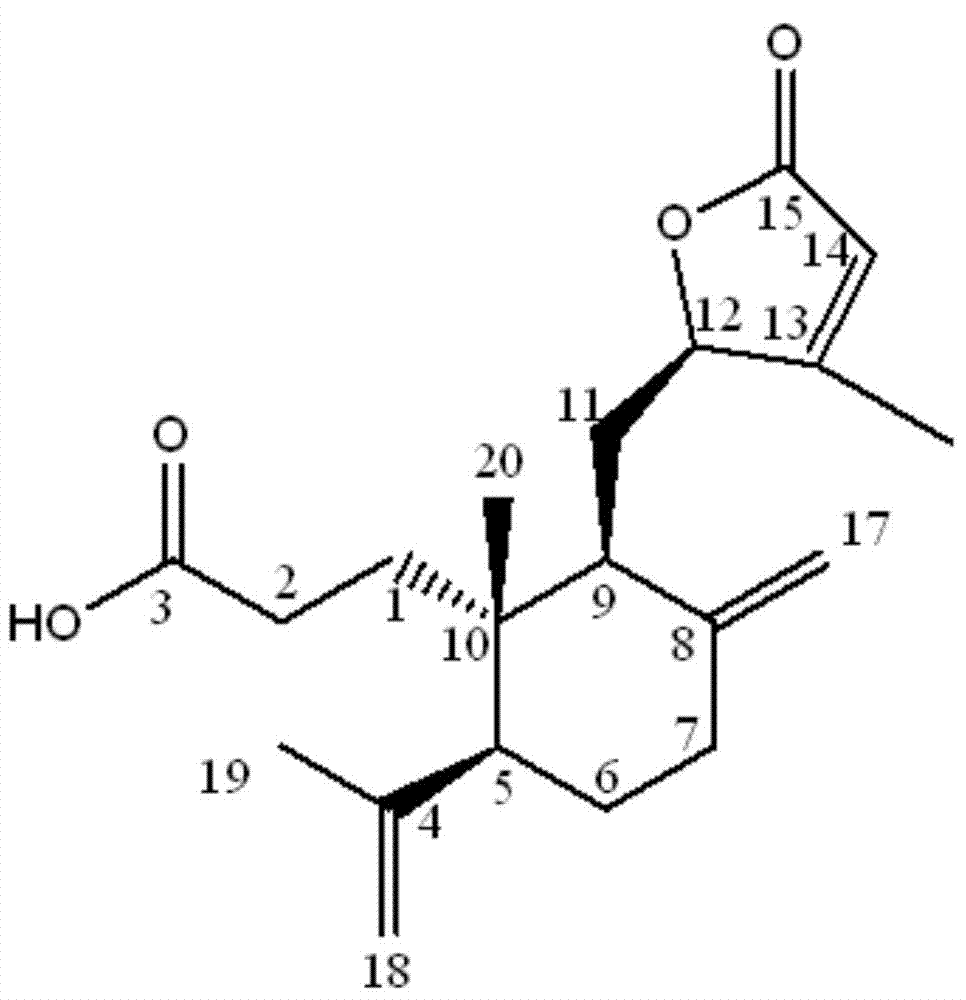
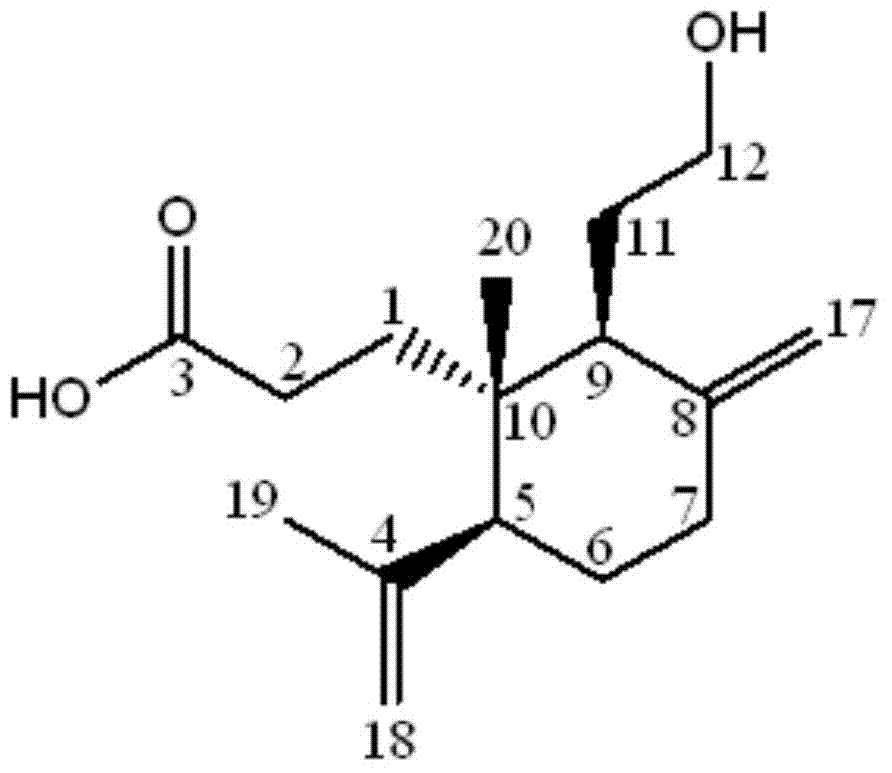
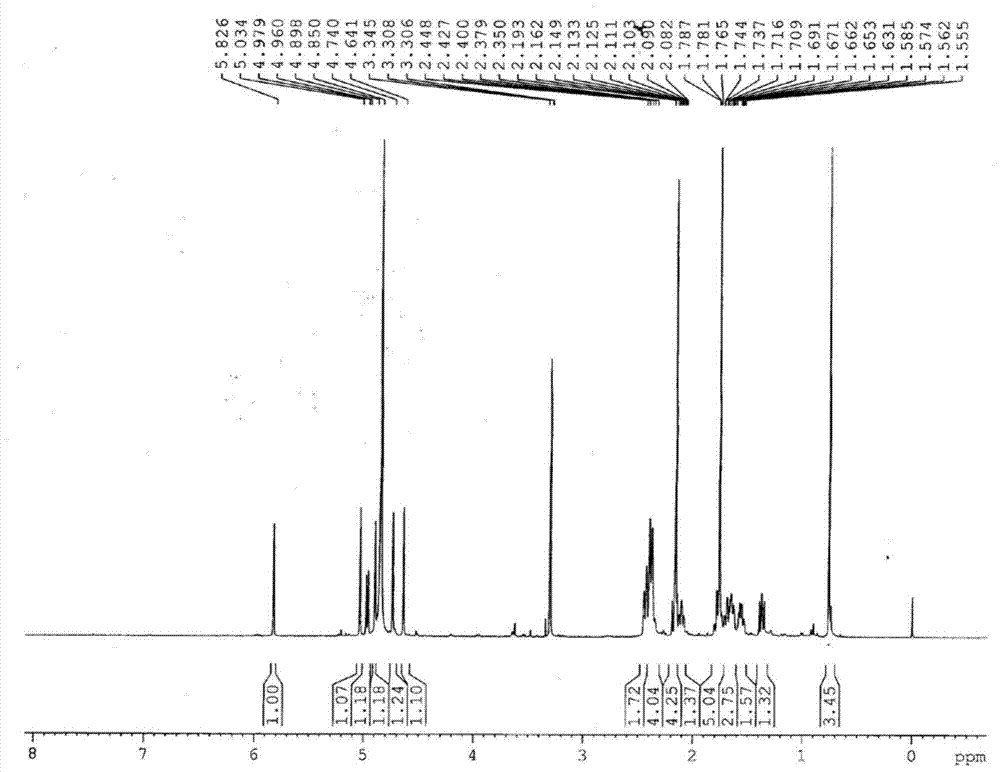
[0042] After testing the physical and chemical properties and spectral data of compounds I and II, their structures were determined (see figure 1 and figure 2 ). The NMR data of compounds Ⅰ and Ⅱ are shown in Table 1 (see Figure 3-Figure 6 ), the high-resolution mass spectrometry data are shown in Table 2.
[0043] Table 1 NMR data of compounds Ⅰ and Ⅱ a
[0044]
[0045] a. 1 The test condition of H-NMR is 600MHz, and the spectroscopic reagent of compound Ⅰ is CD 3 OD, the patterning reagent of compound Ⅱ is Pyr-d; 13 The test condition of C-NMR is 150MHz, and the spectral reagent of compound Ⅰ is CD 3 OD, the patterning reagent of compound II is Pyr-d.
[0046] Table 2 High resolution mass spectrometry data of compounds Ⅰ and Ⅱ
[0047] sample name
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com