Method for preparing 11A, 17A-dihydroxy-pregna-1,4-diene-3,20-dione by enzymatic method
A technology of enzymatic preparation and dihydroxy, which is applied in fermentation and other directions, can solve the problems of low substrate concentration, substrate conversion, low reaction efficiency, etc., and achieve high product yield and good quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 (Preparation of KSDH):
[0032] The KSDH enzyme powder was prepared by conventional methods: The KSDH enzyme powder was derived from Arthrobacter simplex IFO12069 described in the literature J.Biochem.117,1043-1049 and the literature Lett.Appl.Microbiol.44 (2007)563-568. The KSDH gene fragment (synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd.) was ligated with the pET30a plasmid digestion product, and then transformed into the competent E.coli BL21(DE3) strain. The positive clones were screened and inoculated into resistant ones. In liquid LB medium, culture at 37°C to OD600 to 0.8, add inducer IPTG, continue culture for 16 hours, centrifuge to collect the precipitate, add phosphate buffer to suspend, sonicate in an ice water bath for 10 minutes, centrifuge to take the supernatant, freeze Dry to obtain KSDH enzyme powder.
Embodiment 2
[0033] Example 2 (Verification of KSDH catalytic reaction):
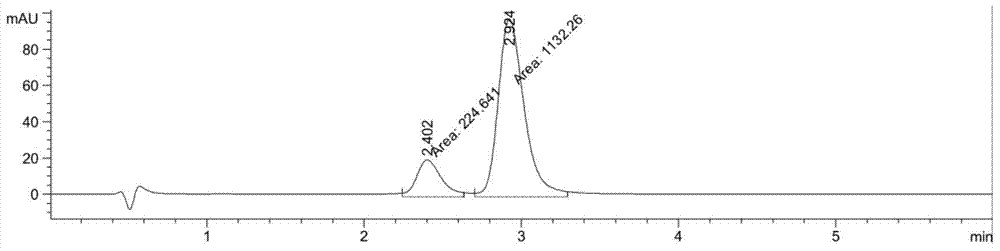
[0034] 10mg of substrate and 40mg of KSDH enzyme powder were added to 2ml pH 7.0 phosphate buffer, stirred at 30°C for 20 hours, and the conversion rate detected by HPLC / MS was 16.5% (such as figure 1 Shown).
Embodiment 3
[0035] Example 3 (temperature optimization):
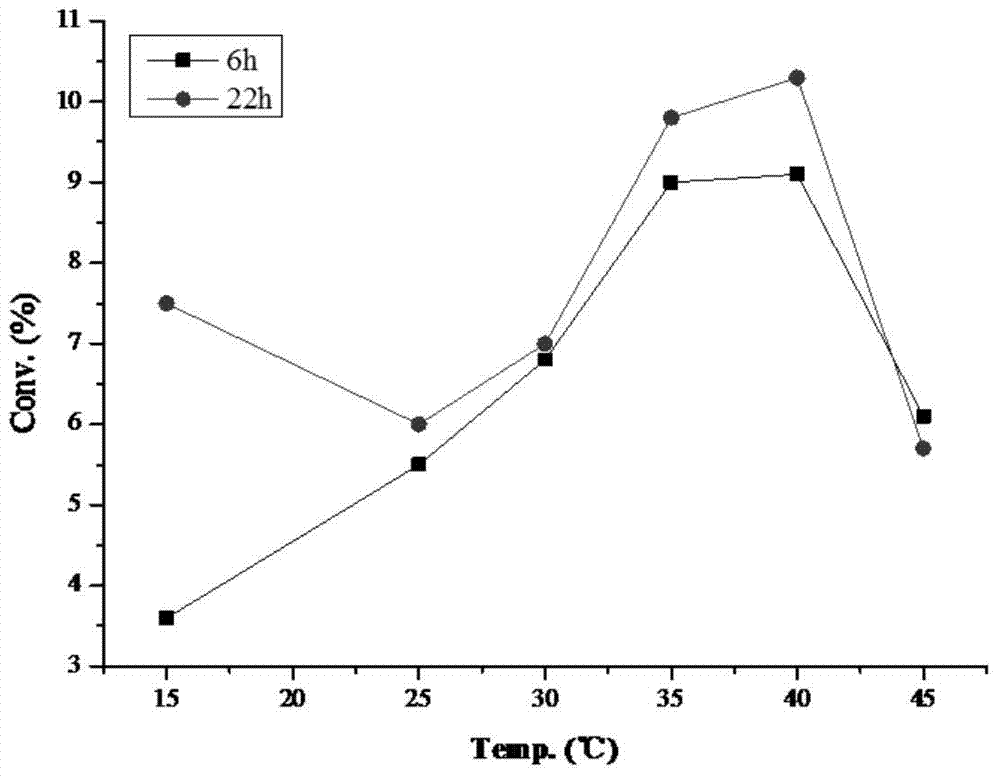
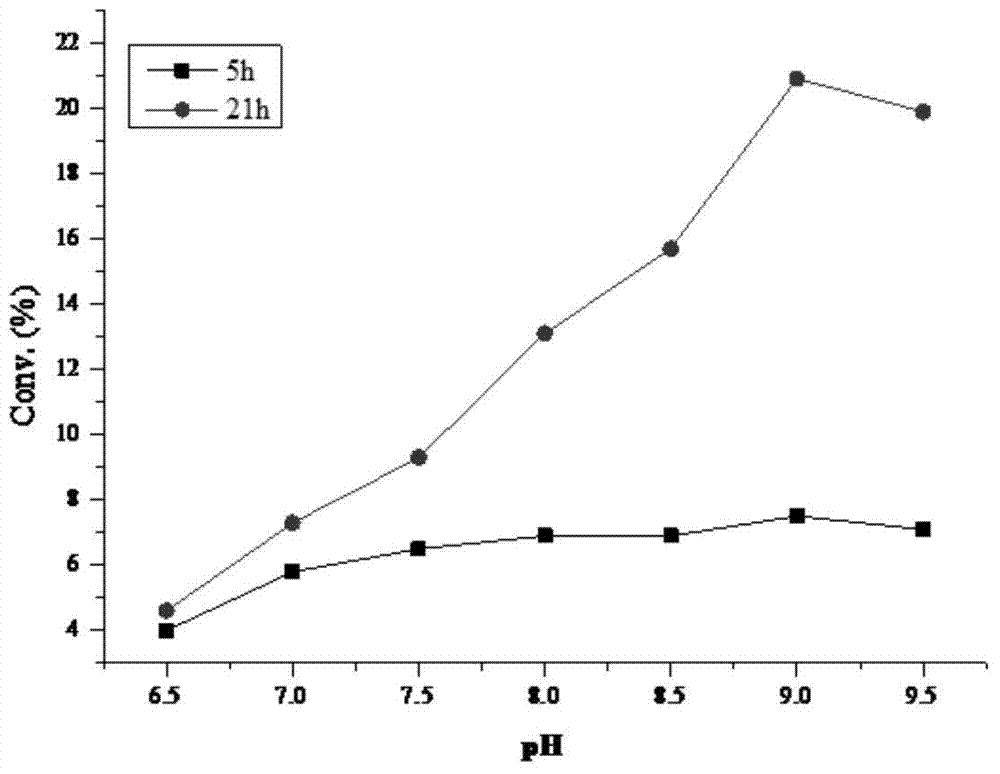
[0036] 20mg of substrate and 20mg of KSDH enzyme powder were added to 2ml pH 7.0 phosphate buffer, stirred at different temperatures for reaction, and the conversion rate detected by HPLC / MS was as follows figure 2 Shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com