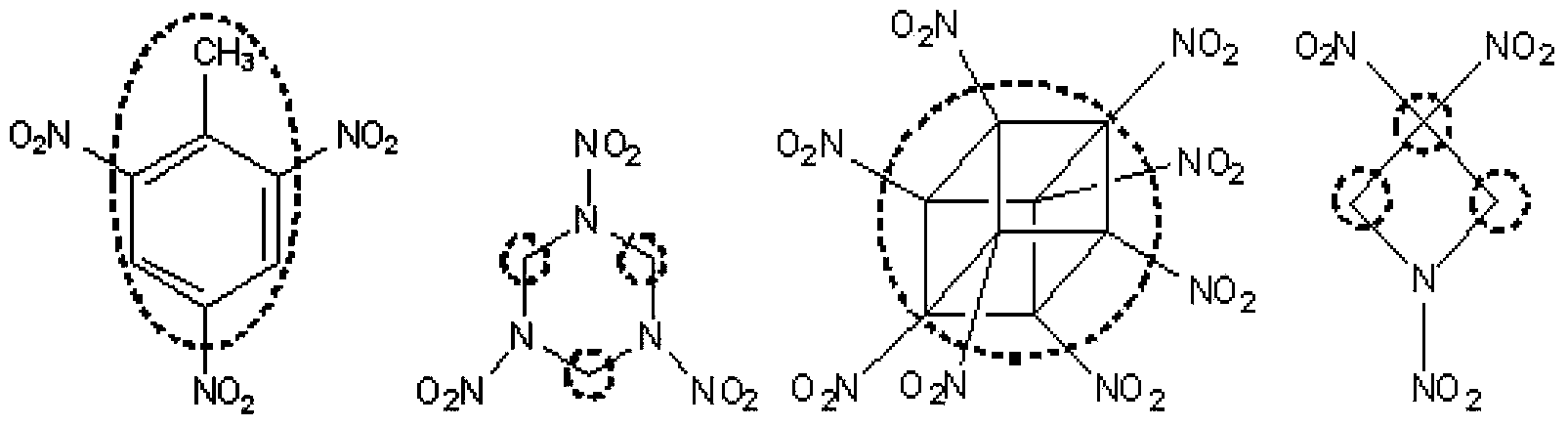
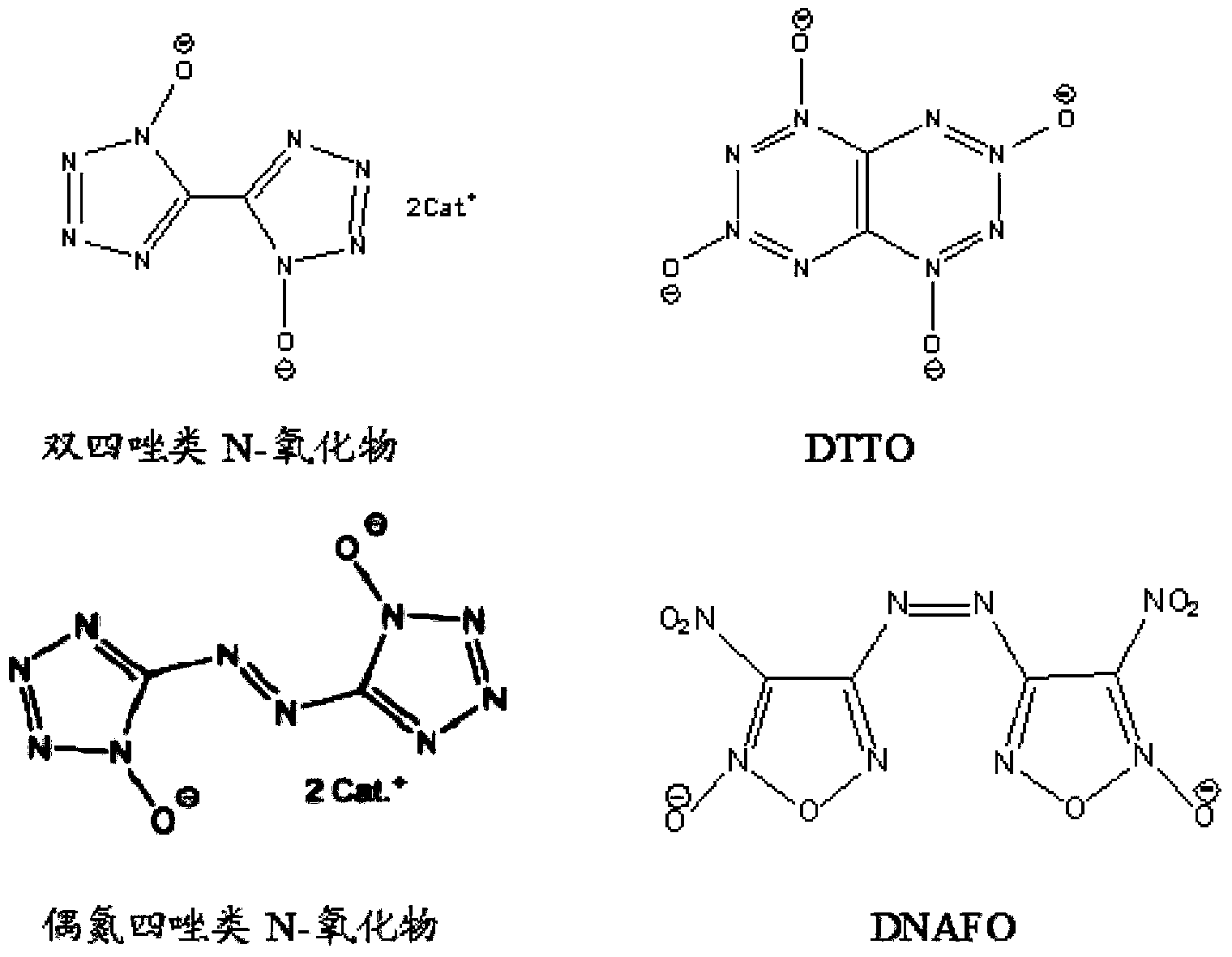
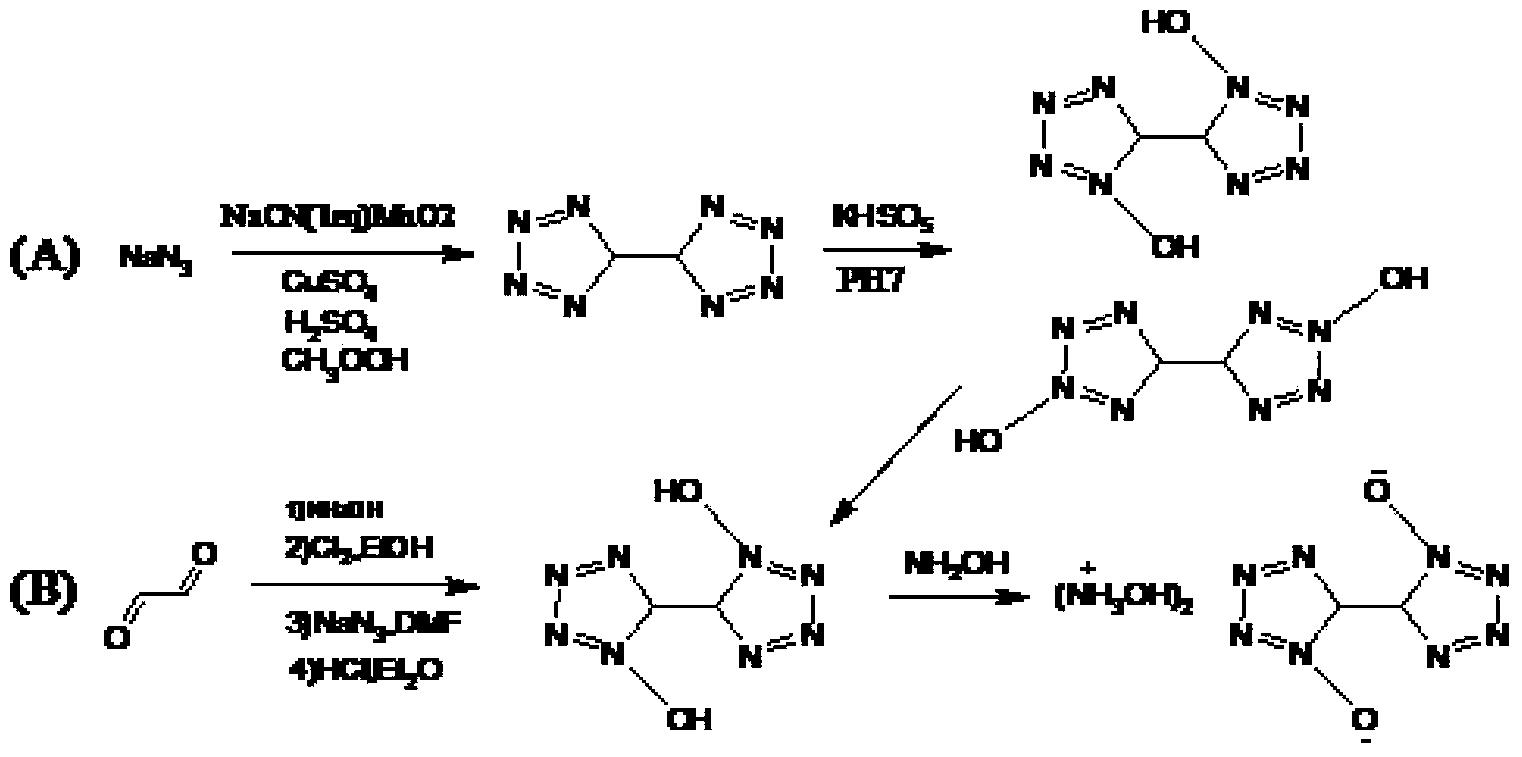
Method of synthesizing 5,5'-bistetrazole-1,1'-dioxodihydroxy ammonium salt
A technology of dioxodihydroxyammonium salt and bistetrazole, which is applied in the direction of organic chemistry, can solve the problems of thermal stability and mechanical stability reduction, aromatic nitro and nitramine toxicity, and increase the difficulty of post-treatment, etc., to achieve The effect of cost reduction, reduction of reaction solvent types, and shortened synthesis cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add 156.0 g (1.0 mol) of dichloroglyoxime to a 2000 ml three-necked flask, and add 1000 ml of methanol solvent. After the methanol solution of dichloroglyoxime was cooled to -40°C, 65.0 g (1.0 mol) of sodium azide was added dropwise to the solution in batches, and stirring was started. After the addition was complete, the reaction was carried out for 0.5 hour and then slowly returned to room temperature. The temperature of the reaction liquid after azidation was lowered to -40° C., hydrogen chloride was passed through, and the reaction was carried out for 1 hour. After purification, the intermediate product 1,1'-dihydroxy-5,5'-bistetrazole was obtained. Dissolve 1,1'-dihydroxy-5,5'-bistetrazole in 500ml of water, add 50g of hydroxide Sodium, stirred and reacted, then 50 g of hydroxylamine hydrochloride was added for reaction to obtain 212.5 g of 5,5'-bistetrazole-1,1'-dioxydihydroxyammonium salt with a yield of 90%.
Embodiment 2
[0038]Example 2: 312.0 g (2 mol) of dichloroglyoxime was added to a 2000 ml three-necked flask, and 1000 ml of propylene glycol solvent was added. After cooling the propylene glycol solution of p-dichloroglyoxime to 0°C, add 117.0 g (1.8 mol) of sodium azide to the solution and start stirring. After the addition was complete, the reaction was carried out for 10 hours and then slowly raised to room temperature. Keep the reaction liquid after azidation at 40°C, add 1 L of 98% concentrated sulfuric acid dropwise, react for 15 hours, and obtain the intermediate product 1,1'-dihydroxy-5,5'-bistetrazole after purification. Dissolve 1,1'-dihydroxy-5,5'-bistetrazole in 1000ml of water, add 100g of sodium hydroxide, stir for reaction, then add 100g of hydroxylamine hydrochloride for reaction to obtain 5,5'-bistetrazole-1,1 401.5 g of '-dioxydihydroxyammonium salt, the yield is 85%.
Embodiment 3
[0040] Add 7.8kg (50mol) of dichloroglyoxime to a 50L reactor, and add 30L of ethanol solvent. After cooling the ethanol solution of dichloroglyoxime to 20°C, start adding 3.8kg (60mol) of sodium azide to the solution and start stirring. After the addition was complete, the reaction was carried out for 24 hours, and the temperature was slowly raised to room temperature. Cool the reaction liquid after azidation to 0°C, add 10L polystyrene sulfonic acid resin, stir for 24 hours, and purify to obtain the intermediate product 1,1'-dihydroxy-5,5'-bistetrazole , Dissolve 1,1'-dihydroxy-5,5'-bistetrazole in 20L of water, add 1kg of sodium hydroxide, stir for reaction, then add 1kg of hydroxylamine hydrochloride for reaction, and obtain 5,5'-bistetrazole-1 , 9.45kg of 1'-dioxydihydroxyammonium salt, the yield was 80%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com