Acridine-1, 2, 3-triazole type compound and preparation method and application thereof
A compound, triazole technology, applied in the field of medicine, achieves good medicinal value and significant anti-tumor activity in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
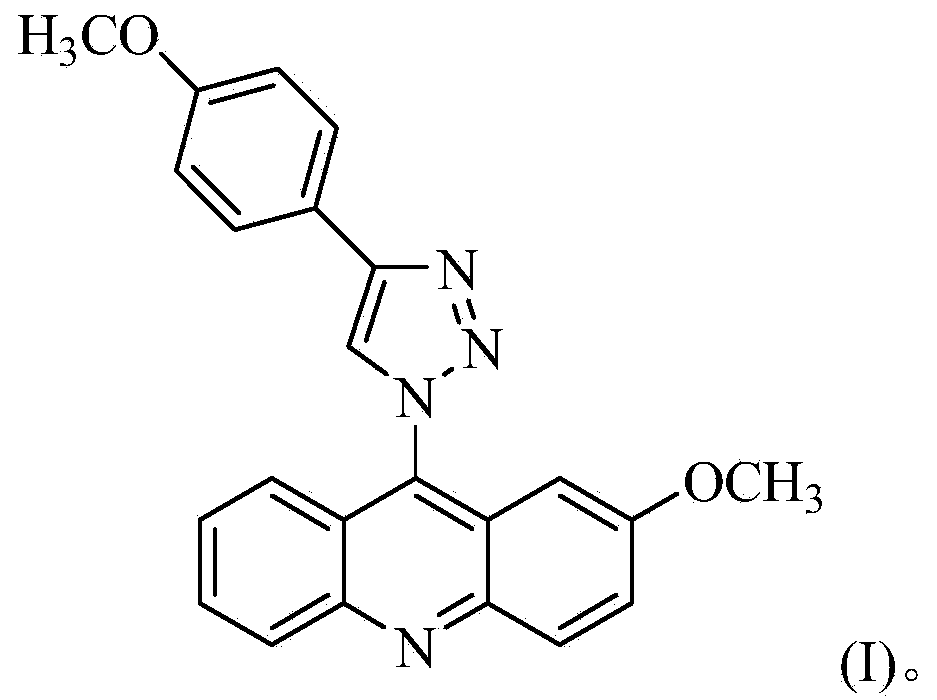
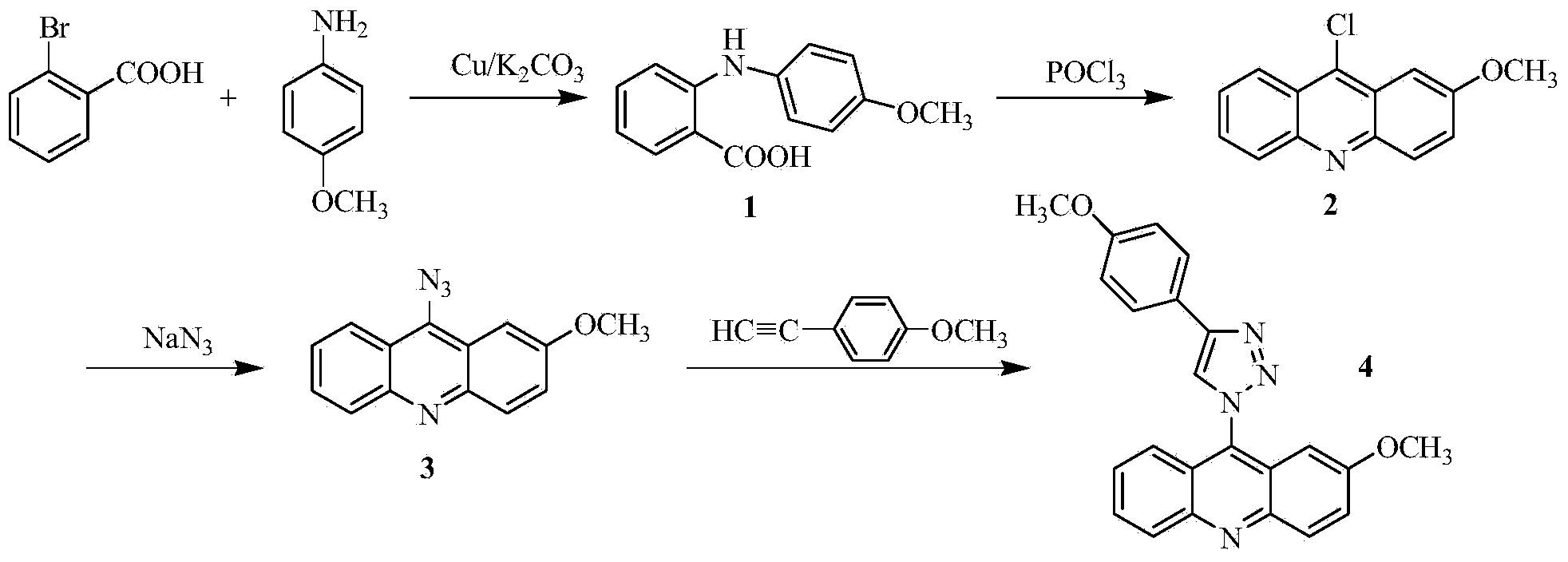
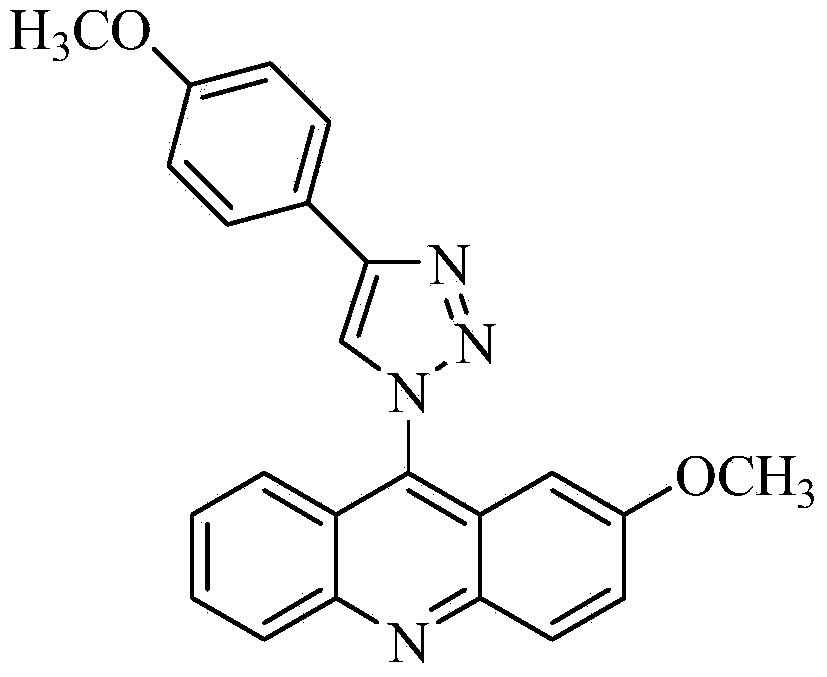
[0024] The preparation of 2-methoxy-9-acridine (p-methoxyphenyl)-1,2,3-triazole, concrete steps are as follows:
[0025] 1) In a 250mL three-necked flask, add 5.20g (26mmol) of o-bromobenzoic acid, 4.19g (34mmol) of p-methoxyaniline, 7.5g (36.2mmol) of potassium carbonate and 0.3g (4.7mmol) of copper powder, and then Add 30 mL of isoamyl alcohol as a solvent, reflux at 140°C and stir for 2 hours. After the reaction, evaporate the solvent under reduced pressure, add 600 mL of water to the obtained residue, stir at 80°C for 20 minutes, filter while hot, wash the filter cake with water, and combine the water layers , the aqueous layer was acidified with concentrated hydrochloric acid to pH = 2, a large amount of light green precipitate was precipitated, filtered with suction, and the obtained solid was recrystallized with chloroform to obtain compound 1 with a yield of 79%;
[0026] 2) In a 100mL round-bottomed flask, add 4.38g (18mmol) of compound 1 and 14.37mL of phosphorus oxy...
Embodiment 2
[0036] Repeat Example 1, the difference is:
[0037] In step 1), change the consumption of potassium carbonate to 5.39g (26mmol), copper powder 0.17g (2.6mmol), replace isoamyl alcohol with n-amyl alcohol, change the temperature during reflux to 150°C, and combine the water The layer was acidified to pH=4 with concentrated hydrochloric acid;
[0038] In step 3), the amount of sodium azide was changed to 0.26g (4mmoL), and the temperature during the reaction was changed to 80°C;
[0039] In step 4), change the volume ratio of tert-butanol to water in the tert-butanol / water solution to 4:6.
[0040] Mass spectrometry, carbon spectrometry and infrared analysis were performed on the separated brown needle-like crystals, and it was determined to be 2-methoxy-9-acridine(p-methoxyphenyl)-1,2,3-triazole.
Embodiment 3
[0042] Repeat Example 1, the difference is:
[0043] In step 1), the temperature at reflux was changed to 160° C., and the combined aqueous layer was acidified to pH=1 with concentrated hydrochloric acid;
[0044] In step 4), the volume ratio of tert-butanol and water in tert-butanol / water solution was changed to 8:2, and the solvent for recrystallization was changed to methanol.
[0045] Mass spectrometry, carbon spectrometry and infrared analysis were performed on the separated brown needle-like crystals, and it was determined to be 2-methoxy-9-acridine(p-methoxyphenyl)-1,2,3-triazole.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com