Method for producing potassium carbonate by using solid wastes containing potassium and chlorine ions
A chloride ion and solid waste technology, which is applied to the removal of solid waste, alkali metal carbonate, chemical instruments and methods, etc., can solve the problems of unreported waste water utilization technology, and achieves low production cost and good operation safety. , the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
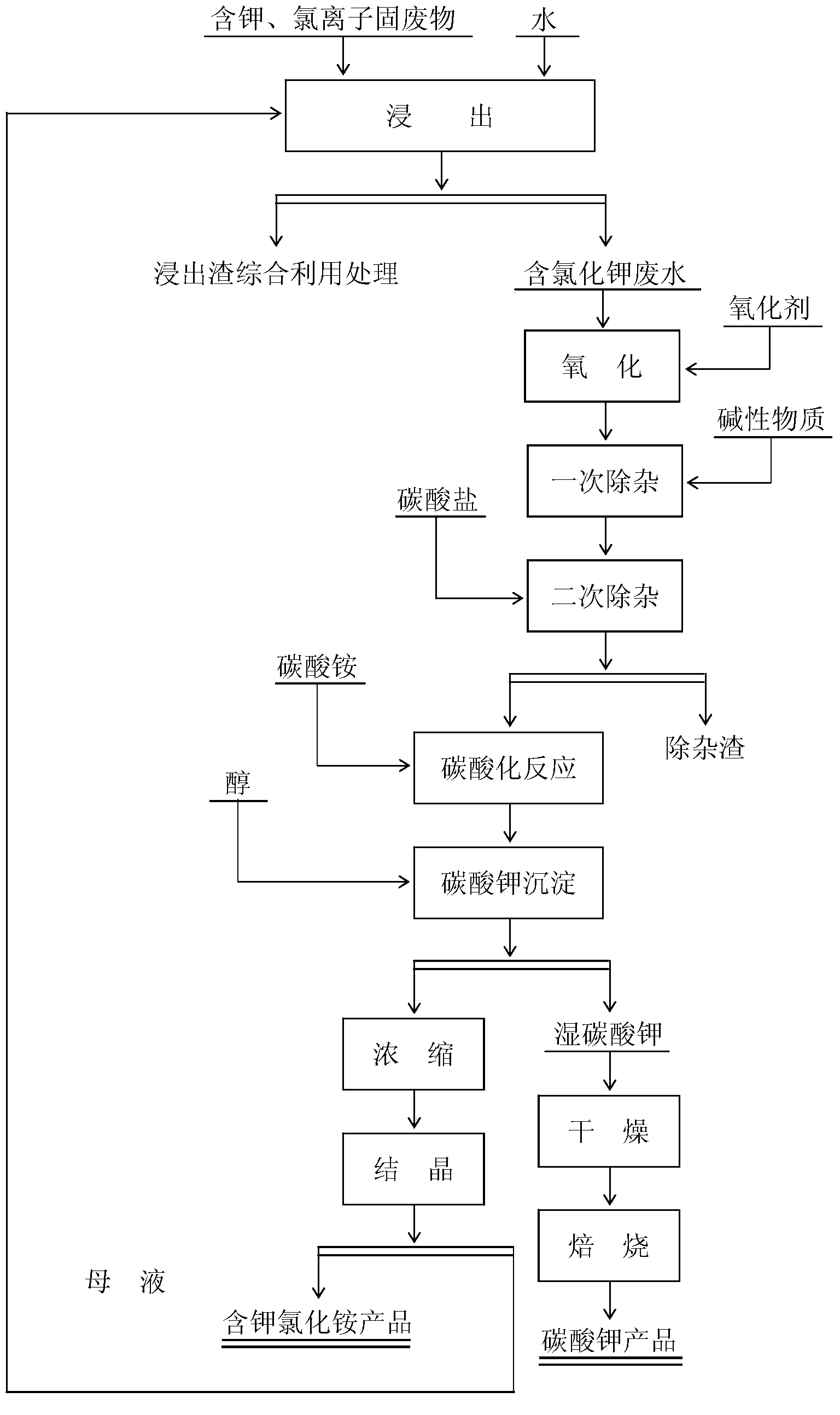
[0048] 1. Remove impurities
[0049] To the leaching wastewater containing potassium and chloride ion solid waste (main component: K 2 O content is 4.7%, Cl ion content is 5.8%), add the potassium permanganate of solution weight 0.3, oxidation reaction 90min, then add unslaked lime (add-on is 0.08% of solution weight) to adjust the pH value=11.5 of solution, After stirring and removing impurities for 60 minutes, add ammonium carbonate (the addition amount is 0.08% of the solution weight) to the solution, stir and remove impurities for 60 minutes, filter, and the filtrate enters the buffer tank for subsequent use.
[0050] 2. Carbonation reaction
[0051] Add the solution in the buffer tank to the reaction tank, and simultaneously add 5.2% ammonium carbonate (the molar ratio of ammonium carbonate to potassium chloride=1.85) of the solution weight. After stirring for 180 min, the solution enters the potassium carbonate precipitation process.
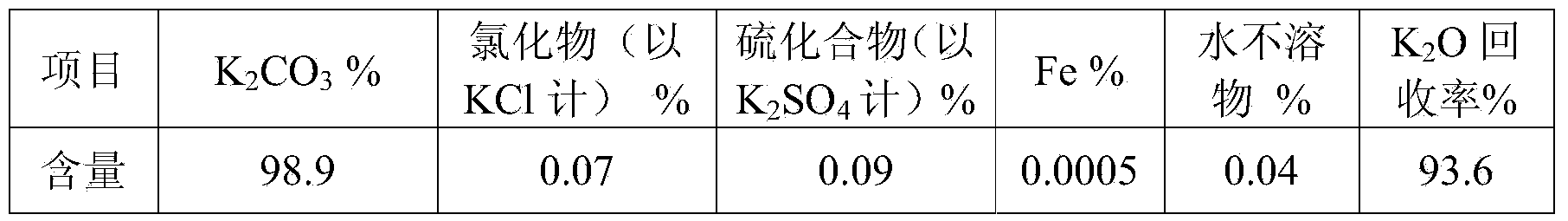
[0052] 3. Potassium carbonate pre...
Embodiment 2
[0063] 1. Remove impurities
[0064] To the leaching wastewater containing potassium and chloride ion solid waste (main component: K 2 O content is 4.9%, Cl ion content is 6.0%), add the hydrogen peroxide of solution weight 4% (hydrogen peroxide concentration is 35%) in), oxidation reaction 180min, then add potassium hydroxide (addition is 0.1% of solution weight) Adjust the pH value of the solution to 13.5, stir to remove impurities for 90 minutes, then add potassium carbonate (the addition amount is 0.15% of the solution weight) to the solution, stir to remove impurities for 70 minutes, filter, and the filtrate enters the buffer tank for later use.
[0065] 2. Carbonation reaction
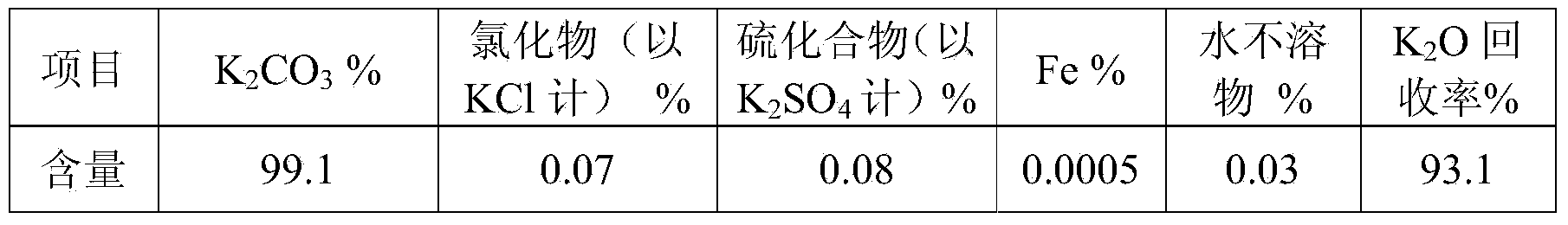
[0066] Add the solution in the buffer tank to the reaction tank, and add 5.8% ammonium carbonate (the molar ratio of ammonium carbonate to potassium chloride=2.05) of the solution at the same time. After stirring for 150 minutes, the solution enters the potassium carbonate precipitation process....
Embodiment 3
[0078] 1. Remove impurities
[0079] To the leaching wastewater containing potassium and chloride ion solid waste (main component: K 2 O content is 9.2%, Cl ion content is 11.6%), add the sodium hypochlorite of solution weight 0.6% in), oxidation reaction 120min, then add sodium carbonate (addition is 1.5% of solution weight) and adjust the pH value=14 of solution, stir After removing impurities for 90 minutes, add ammonium carbonate (the addition amount is 0.08% of the solution weight) to the solution, stir and remove impurities for 90 minutes, filter, and the filtrate enters the buffer tank for subsequent use.
[0080] 2. Carbonation reaction
[0081] Add the solution in the buffer tank to the reaction tank, and add 11.7% ammonium carbonate (the molar ratio of ammonium carbonate to potassium chloride=2.5) of the solution weight at the same time. After stirring for 240min, the solution enters the potassium carbonate precipitation process.
[0082] 3. Potassium carbonate pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com