Method for catalysis synthesis of (Z)-2-acetylamino methyl cinnamate through DCC/DMAP
A technology of methyl cinnamate and acetamido, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve the problems of complicated operation, only yield, high solubility and difficult to eliminate, and achieves simple post-processing. , the effect of easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
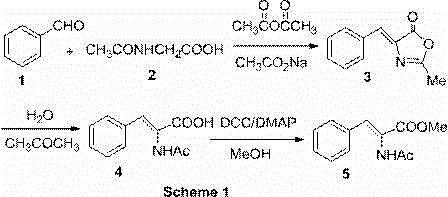
[0023] , Synthesis of 3-phenylacrylic acid azlactone (3)
[0024] Take 11.75 g of N-acetylaminoglycine, 5 g of sodium acetate, and 13.25 g of benzaldehyde into a 250 ml round-bottomed flask, then add 35 ml of acetic anhydride, reflux at 100 °C for 3 h, cool to room temperature, and precipitate a large amount of brown-yellow solid , placed in a refrigerator at 4°C overnight, filtered through a sand core funnel, washed with dilute acetic acid aqueous solution (20 ml×3), then washed with cold water until it was a yellow needle-like solid, and dried to obtain 13.65 g of a yellow compound 3 , and the yield was 73.8%.
[0025] 2. Preparation of (Z)-2-acetylaminocinnamic acid (4)
[0026] Transfer 13 g of the product obtained in the previous step to a 500 ml round bottom flask, add 50 ml of water, 100 ml of acetone, reflux at 80 °C for 4 h, evaporate to dryness with a rotary evaporator, add 200 ml of distilled water, heat to 100 °C and After hot suction filtration, the filtrate ...
Embodiment 2
[0035] Same as Example 1, but the volume ratio of the mixture of methanol and dichloromethane is 0.5:1, and the weight ratio of N,N-dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) is 70:1. The yield was 68.0%, and the purity was greater than 99%.
Embodiment 3
[0037] Same as Example 1, but the volume ratio of the mixture of methanol and dichloromethane is 1:0.5, the weight ratio of N,N-dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) is 90:1. The yield was 68.3%, and the purity was greater than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com